3.4: Cyclic Ethers - Epoxides
- Page ID
- 469370
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
Synthesis of Epoxides
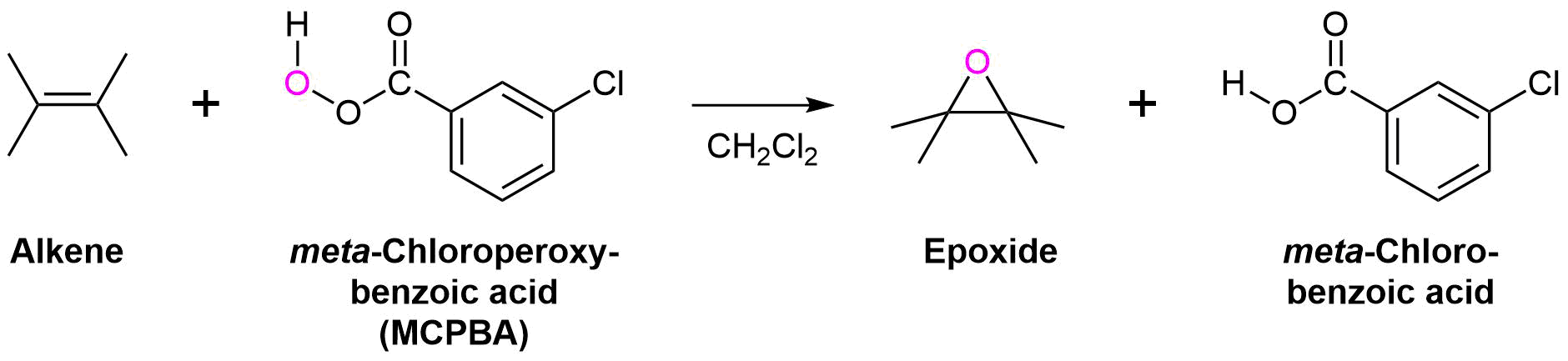
Epoxides are typically prepared by the reaction of an alkene and a peroxycarboxylic acid (RCO3H), such as meta-chloroperbenzoic acid (MCPBA). The peroxycarboxylic acid has the unique property of having an electropositive oxygen atom on the CO3H group. The reaction is initiated by the electrophilic oxygen atom reacting with the nucleophilic carbon-carbon double bond. The mechanism involves a concerted reaction with a four-part, circular transition state. The result is that the originally electropositive oxygen atom ends up in the oxacyclopropane ring and the CO3H group becomes CO2H.
General Reaction
Stereochemical Consideration
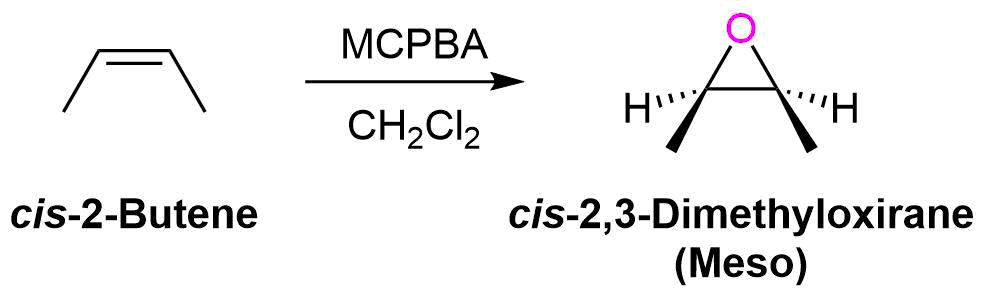
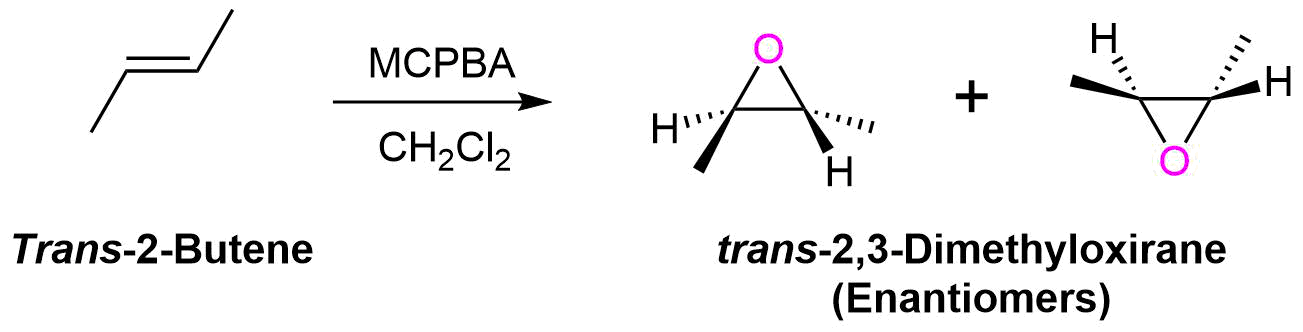
The oxygen addition to the alkene is a syn addition and is stereospecific. The substituents of a cis-alkene will appear in the cis configuration on the epoxide ring. Likewise, Substituents of a trans-alkene will appear in the trans configuration on the epoxide ring. During this reaction the sp2 hybridized carbons of the alkene are converted to sp3 hybridized carbons in the epoxide. Thus, there is the possibility of two new chiral carbons forming in the epoxide product. In most cases, epoxidation of an alkene will product a mixture of enantiomers in the product, due to the electrophilic oxygen being able to attack from above or below the plane of the alkene. One major exception is the epoxidation of symmetrical cis-alkene which produces a meso compound product.
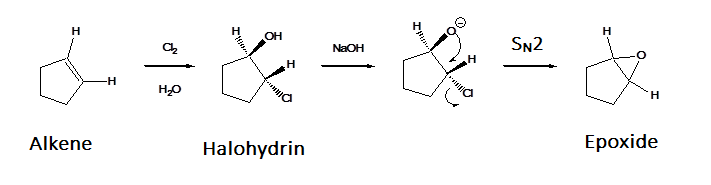
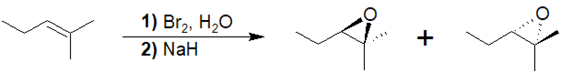
Treatment of an alkene with X2 & H2O creates a halohydrin. When a halohydrin is treated with a base the alcohol is deprotonated to from an alkoxide. This causes an intramolecular Williamson ether synthesis to produce an epoxide along with the elimination of HX. Note, that this reaction generally forms a mixture of enantiomeric products unless a meso compound is produced.
1) What reagents would you use to perform the following transformation?
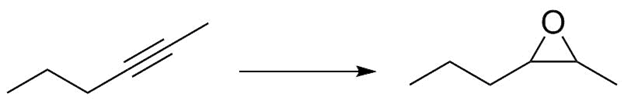
2) Predict the products of the following reactions:
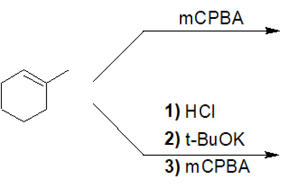
3) Predict the product of the following reaction. Be sure to include proper stereochemistry.
- Answer
-
1) Lindlar's catalyst reduces alkynes to cis/Z alkenes. This stereochemistry is retained after epoxidation.
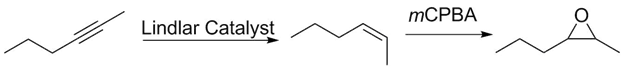
2)
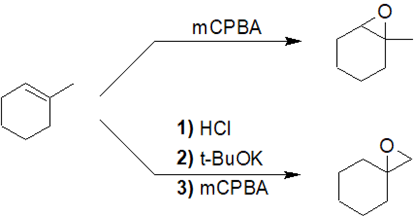
3)
Contributors and Attributions
William Reusch, Professor Emeritus (Michigan State U.), Virtual Textbook of Organic Chemistry
John D. Robert and Marjorie C. Caserio (1977) Basic Principles of Organic Chemistry, second edition. W. A. Benjamin, Inc. , Menlo Park, CA. ISBN 0-8053-8329-8. This content is copyrighted under the following conditions, "You are granted permission for individual, educational, research and non-commercial reproduction, distribution, display and performance of this work in any format."

