3.3: Reactions of Ethers- Acidic Cleavage
- Page ID
- 469369
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Ethers are known to be unreactive towards most reagents which makes them excellent reaction solvents. The most common reaction of ethers is cleavage of the C–O bond by using strong acids. During acidic cleavage the ether oxygen is protonated to form a good leaving groups which can be eliminated as part of an SN2, SN1, or E1 reaction mechanism. The mechanistic pathway is primarily determined by the strong acid used and the type of substituents attached to the ether.
Acidic Cleavage of Ethers
Aqueous solutions of HBr or HI (but not HCl) tend to cleave ethers into alcohol and an alkyl halide product by either an SN2 or SN1 mechanism. If the ether is attached to only primary, secondary, or methyl alkyl groups, a selective cleavage will typically take place using an SN2 mechanism. First, the strong acid protonates the ether oxygen. Then resulting halide conjugate base attacks the protonated ether at the less sterically hindered alkyl substituent forming a halogen product. The ether's more sterically hindered alkyl substituent is ejected as a leaving group and forms an alcohol product. The example below show that when ethyl isopropyl ether is cleaved with hydrobromic acid the products isopropyl alcohol and bromoethane are produced. The bromide nucleophile preferably attacks the ether's ethyl substituent because it is less hindered (1o) than the isopropyl substituent (2o).
It is important to note that a phenyl substituent on an ether is not capable of participating in the SN2 reaction of an acidic cleavage. If a phenyl group is present it will become a phenol in the product due to the halide nucleophile preferably attacking the other alkyl substituent.
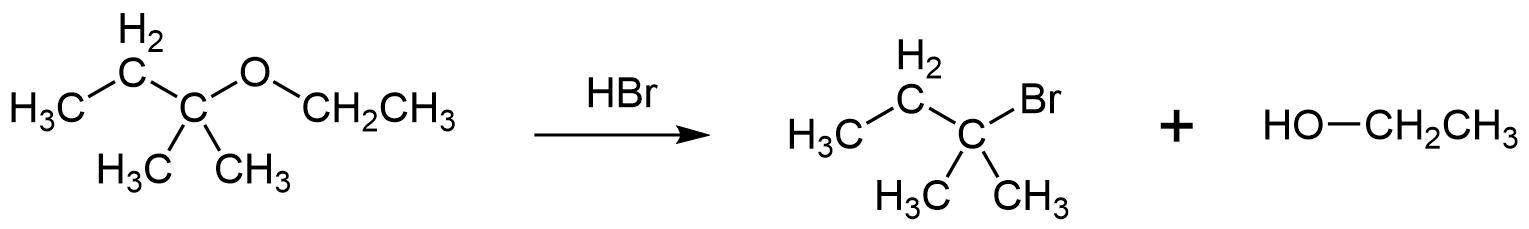
When using HBr or HI, the acidic cleavage of ethers with tertiary, benzylic, or allylic substituents tend to occur by an SN1 mechanism. The ability of these substituents to produce relatively stable carbocations promotes the SN1 mechanism. The change in mechanism causes the tertiary, benzylic, or allyic group to preferably become the halogen product of the acidic cleavage. This makes the ether's other alkyl substituent become the alcohol product.
When using a strong acid whose conjugate base is a poor nucleophile, such as trifluoroacetic acid (CF3CO2H), for the the acidic cleavage of an ether with a tertiary alkyl substituent, the mechanism will often be E1. In this case the tertiary alkyl substituent will lose an adjacent hydrogen to form an alkene product. The ether's other alkyl substituent will form an alcohol product.
Predict the products of the following reaction:
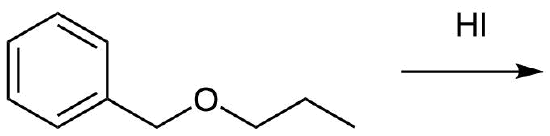
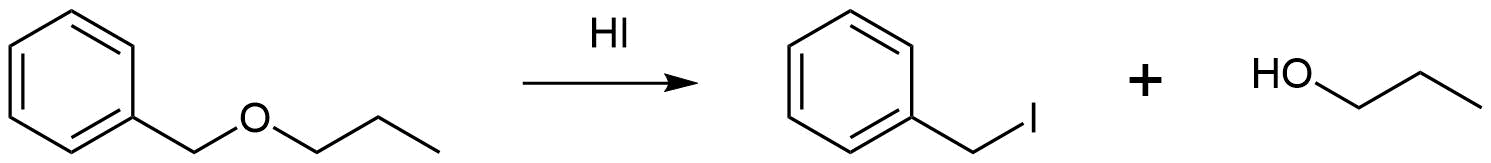
- Answer
-
Analysis: When considering the acidic cleavage of ethers it is important to realize that SN2, SN1, or E1 reactions are possible depending on the conditions. First, identify if the ether has a substituent which can easily form a carbocation: tertiary, benzylic, or allylic substituents. If none of these substituents are present the reaction will most likely be SN2. However, if one of the substituents is present the reaction will most likely be SN1 or E1. Next, identify if the reaction conditions will allow for an E1 reaction. These are the presence of a tertiary alkyl substituent on the ether along with the use of a strong acid which has a poor nucleophile as a conjugate base. If this set of conditions is not present the reaction will most likely be SN1.
For a SN2 reaction: The less hindered alkyl substituent of the ether will become a halogen. The other alkyl substituent will become an alcohol.
For a SN1 reaction: The substituent which easily forms a carbocation will become a halogen and the other alkyl substituent will become an alcohol.
For an E1 reaction: The tertiary alkyl substutent will form an alkene and the other alkyl substiutent will become an alcohol.
For the reaction proposed above, the reactant contains a benzylic substituent so the reaction will most likely be SN1. Consequently, the benzylic substituent will become and Iodide product and the propyl substituent will become an alcohol.
Solution
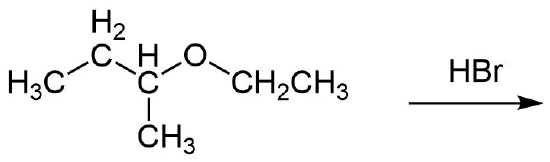
1) Predict the product of the following reactions:
a)
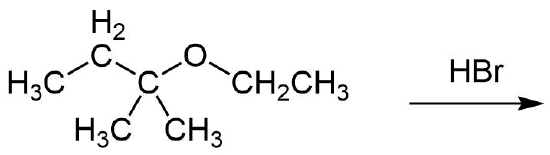
b)
2) Please draw the mechanism for the following reaction:
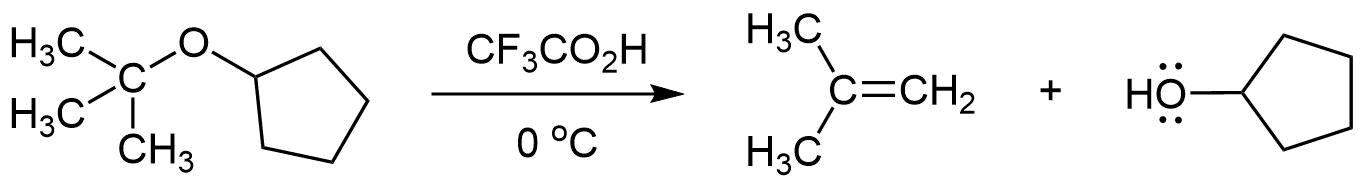
3) Why is HCl less effective at cleaving ethers than HBr or HI?
- Answer
-
1)
a)
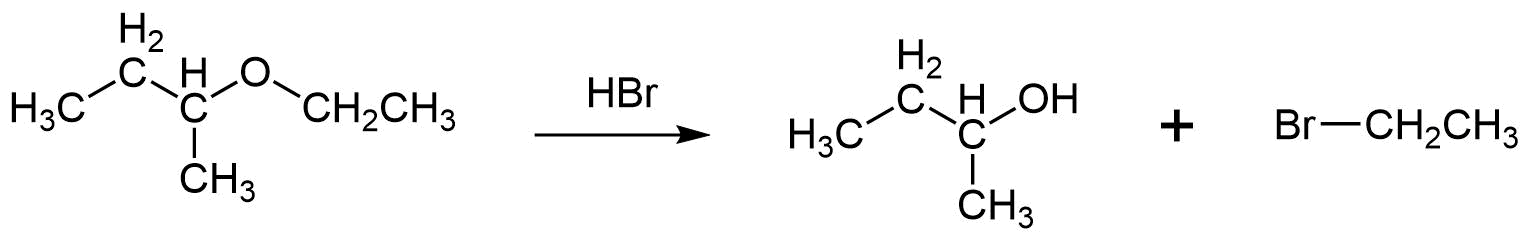
b)
2)
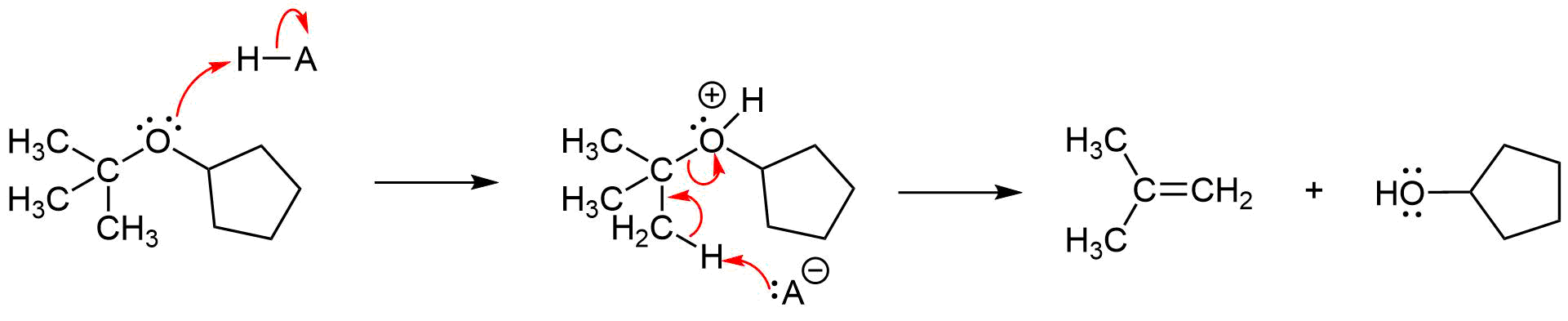
3) HCl's conjugate base Cl- is a poor nucleophile when compared to Br- and I-. The chloride anion is not a strong enough nucleophile to promote the acidic cleavage of ethers.
Contributors and Attributions
William Reusch, Professor Emeritus (Michigan State U.), Virtual Textbook of Organic Chemistry

