By being a reactive intermediate of the electrophilic addition mechanism, the stability of a carbocation has a direct effect on the reaction. The critical question now becomes, what stabilizes a carbocation?
A positively charged species such as a carbocation is very electron-poor, and thus anything which donates electron density will help to stabilize it. Conversely, a carbocation will be destabilized by an electron withdrawing group.

electron donating group stabilizes a carbocation |

electron withdrawing group destabilizes a carbocation |
Extensive experimental evidence has shown that a carbocation becomes more stable as the number of alkyl substituents increases. Carbocations can be given a designation based on the number of alkyl groups attached to the carbocation carbon. Three alkyl groups is called a tertiary (3o) carbocation, 2 alkyl groups is called secondary (2o), and 1 alkyl group is called primary (1o). No alkyl groups are attached (3 hydrogen substituents) is called a methyl carbocation.
The overall order of stability is as follows:
_to_primary_to_secondary_to_tertiary_(most_stable).svg?revision=1)
Alkyl groups stabilized carbocations for two reasons. The first is through inductive effects. As discussed in Section 2-1, inductive effects occur when the electrons in covalent bonds are shifted towards an nearby atom with a higher electronegativity. In this case, the positively charged carbocation draws in electron density from the surrounding substituents thereby gaining stabilization by slightly reducing its positive charge. Alkyl groups are more effective at inductively donating electron density than a hydrogen because they are larger, more polarizable, and contain more bonding electrons. As more alkyl groups are attached to the carbocation more inductive electron donation occurs and the carbocation becomes more stable.
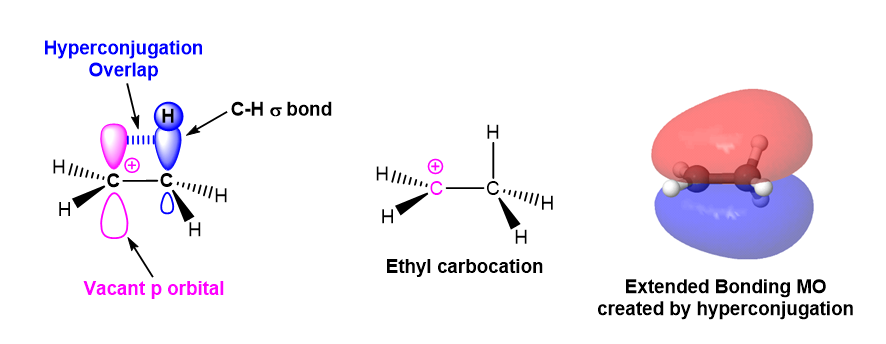
The second reason alkyl groups stabilize carbocations is through hyperconjugation. As previously discussed in Section 7.6, hyperconjugation is an electron donation that occurs from the parallel overlap of p orbitals with adjacent hybridized orbitals participating in sigma bonds. This electron donation serves to stabilize the carbocation. As the number of alkyl substituents increases, the number of sigma bonds available for hyperconjugation increases, and the carbocation tends to become more stabilized.
In the example of ethyl carbocation shown below, the p orbital from a sp2 hybridized carbocation carbon involved interacts with a sp3 hybridized orbital participating in an adjacent C-H sigma bond. Electron density from the C-H sigma bond is donated into carbocation's p orbital providing stabilization.
The molecular orbital of the ethyl carbocation shows the interaction of electrons in methyl group's C-H sigma bonds with the adjacent empty p orbital from the carbocation. The interaction creates a bonding molecular orbital which extends over the three atom chain (C-C-H) involved in hyperconjugation. The expanded molecular orbital helps to stabilize the carbocation.
It is not accurate to say, however, that carbocations with higher substitution are always more stable than those with less substitution. Just as electron-donating groups can stabilize a carbocation, electron-withdrawing groups act to destabilize carbocations. Carbonyl groups are electron-withdrawing by inductive effects, due to the polarity of the C=O double bond. It is possible to demonstrate in the laboratory that carbocation A below is more stable than carbocation B, even though A is a primary carbocation and B is secondary.

The difference in stability can be explained by considering the electron-withdrawing inductive effect of the ester carbonyl. Recall that inductive effects - whether electron-withdrawing or donating - are relayed through covalent bonds and that the strength of the effect decreases rapidly as the number of intermediary bonds increases. In other words, the effect decreases with distance. In species B the positive charge is closer to the carbonyl group, thus the destabilizing electron-withdrawing effect is stronger than it is in species A.
In the next chapter we will see how the carbocation-destabilizing effect of electron-withdrawing fluorine substituents can be used in experiments designed to address the question of whether a biochemical nucleophilic substitution reaction is SN1 or SN2.
Stabilization of a carbocation can also occur through resonance effects, and as we have already discussed in the acid-base chapter, resonance effects as a rule are more powerful than inductive effects. Consider the simple case of a benzylic carbocation:

This carbocation is comparatively stable. In this case, electron donation is a resonance effect. Three additional resonance structures can be drawn for this carbocation in which the positive charge is located on one of three aromatic carbons. The positive charge is not isolated on the benzylic carbon, rather it is delocalized around the aromatic structure: this delocalization of charge results in significant stabilization. As a result, benzylic and allylic carbocations (where the positively charged carbon is conjugated to one or more non-aromatic double bonds) are significantly more stable than even tertiary alkyl carbocations.

Because heteroatoms such as oxygen and nitrogen are more electronegative than carbon, you might expect that they would by definition be electron withdrawing groups that destabilize carbocations. In fact, the opposite is often true: if the oxygen or nitrogen atom is in the correct position, the overall effect is carbocation stabilization. This is due to the fact that although these heteroatoms are electron withdrawing groups by induction, they are electron donating groups by resonance, and it is this resonance effect which is more powerful. (We previously encountered this same idea when considering the relative acidity and basicity of phenols and aromatic amines in section 7.4). Consider the two pairs of carbocation species below:


In the more stable carbocations, the heteroatom acts as an electron donating group by resonance: in effect, the lone pair on the heteroatom is available to delocalize the positive charge. In the less stable carbocations the positively-charged carbon is more than one bond away from the heteroatom, and thus no resonance effects are possible. In fact, in these carbocation species the heteroatoms actually destabilize the positive charge, because they are electron withdrawing by induction.
Finally, vinylic carbocations, in which the positive charge resides on a double-bonded carbon, are very unstable and thus unlikely to form as intermediates in any reaction.

a vinylic carbocation (very unstable)
Example \(\PageIndex{1}\)
In which of the structures below is the carbocation expected to be more stable? Explain.

- Answer
-
In the carbocation on the left, the positive charge is located in a position relative to the nitrogen such that the lone pair of electrons on the nitrogen can be donated to fill the empty orbital. This is not possible for the carbocation species on the right.

Example \(\PageIndex{2}\)
Draw a resonance structure of the crystal violet cation in which the positive charge is delocalized to one of the nitrogen atoms.
- Answer

When considering the possibility that a nucleophilic substitution reaction proceeds via an SN1 pathway, it is critical to evaluate the stability of the hypothetical carbocation intermediate. If this intermediate is not sufficiently stable, an SN1 mechanism must be considered unlikely, and the reaction probably proceeds by an SN2 mechanism. In the next chapter we will see several examples of biologically important SN1 reactions in which the positively charged intermediate is stabilized by inductive and resonance effects inherent in its own molecular structure.
Example \(\PageIndex{3}\)
State which carbocation in each pair below is more stable, or if they are expected to be approximately equal. Explain your reasoning.
_tertiary_vs_secondary_carbocation%252C_b)_tertiary_versus_secondary_carbocation%252C_c)_secondary_vs_secondary_carbocation%252C_d)_carbocation_adjacent_to_nitrogen_atom_versus_tertiary_carbocation%252C_and_e)_allylic_versus_tertiary_carbocation.svg?revision=1)
- Answer
-
- 1 (tertiary vs. secondary carbocation)
- 1 (tertiary vs. secondary carbocation)
- 2 (positive charge is further from electron-withdrawing fluorine)
- 1 (lone pair on nitrogen can donate electrons by resonance)
- 1 (allylic carbocation – positive charge can be delocalized to a second carbon)



