4.2: Molecular Orbital Theory and Lewis acid-base reactions
- Page ID
- 402349
A brief review of the Lewis acid-base theory
In this chapter we will discuss Lewis-acid base reactions from the perspective of molecular orbital theory. The Lewis acid-base concept has been developed by Gilbert Lewis (Fig. 4.1.1).

https://commons.wikimedia.org/wiki/F...wton-Lewis.jpg)
It is a very general acid-base concept, and includes the Broensted and Arrhenius acid-base concepts. It is very important for coordination chemistry. Let us briefly review what a Lewis acid, a Lewis base, and a Lewis acid-base reaction is.
A Lewis acid is defined as an electron pair acceptor.
A Lewis acid is an electron pair acceptor
A Lewis base is defined as an electron pair donor.
A Lewis base is an electron pair donor
An example of a Lewis acid is BH3, and an example for a Lewis base is NH3. What happens in a Lewis-acid base reaction?
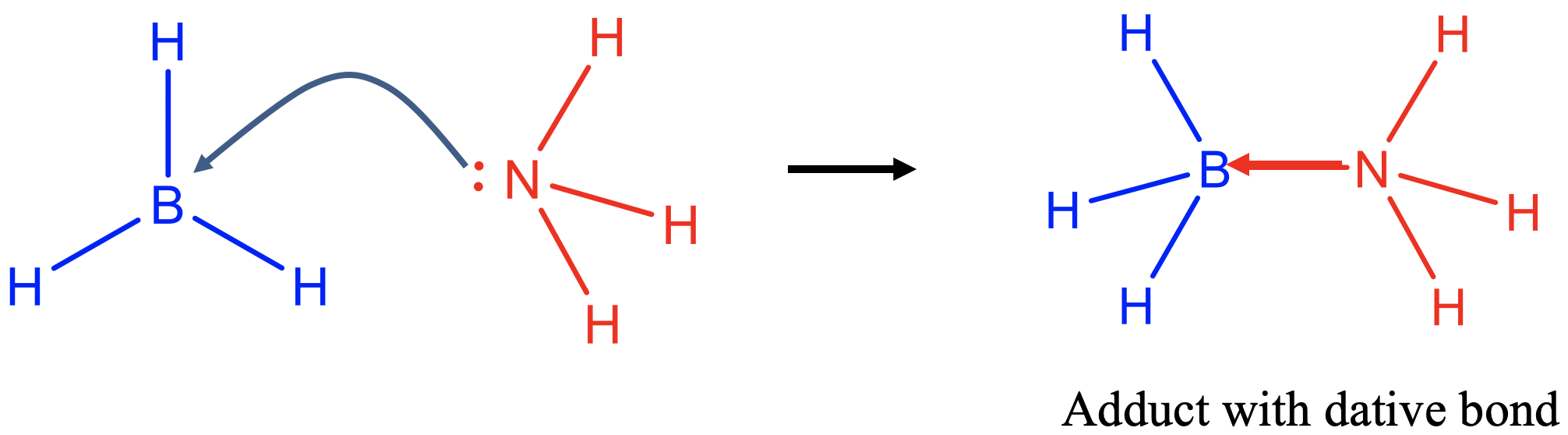
The Lewis base donates an electron pair to form a covalent bond with the Lewis acid (Fig. 4.1.2). A covalent bond formed in a Lewis acid-base reaction is usually called a dative bond because both electrons in the covalent bond come from a single partner. In a "conventional" covalent bond both partners contribute one electron to the covalent bond. There is however no fundamental difference between a “conventional” covalent bond and a dative covalent bond, it is just a matter of perspective. To indicate a dative bond one can draw an arrow pointing from the donor to the acceptor atom, instead of just a line. The reaction product of a Lewis acid-base reaction is called an adduct.
As mentioned previously, the Lewis acid-base concept is quite general and can explain the bonding in quite different compounds. It includes the Broensted acid-base concept, meaning that any Broensted acid is also a Lewis acid, and any Broensted base is also a Lewis base. However, the reverse is not true. Not every Lewis acid is a Broensted acid, and not every Lewis base is a Broensted base.
Here are a few examples that can illustrate the generality of the Lewis acid-base concept.
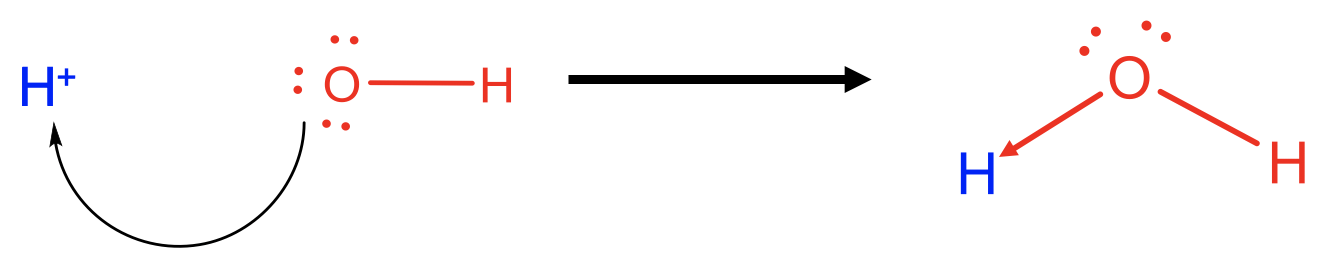
For example, an H+ ion is a Lewis acid and an OH- anion is a Lewis base, and the two can react to form water as a Lewis-acid base adduct. In this case one of the O-H bonds in the water molecule would be viewed as a dative bond, while the other one would be viewed as a “regular” covalent bond. Of course, there is no actual difference between the two bonds, we just have a different perspective on them. The same reaction could also be viewed as a Broensted acid base reaction with the OH- ion as the proton acceptor, and the H+ ion as the proton donor.
The Lewis acid-base concept also explains bonding in coordination compounds, and the formation of coordination compounds from metal ions and ligands. The ligand is the Lewis base and the metal ion is the Lewis acid, the coordination compound is the Lewis acid-base adduct. The bond between the metal ion and ligand is a dative bond pointing from the ligand to the metal.
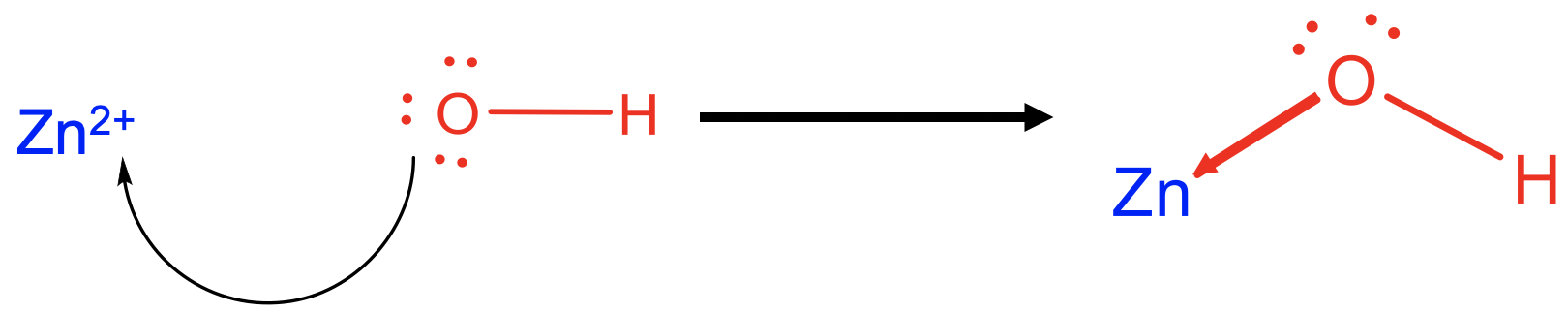
For example, Zn2+ acts as a Lewis acid when reacting with 4 OH- as a Lewis base to form tetrahydroxo zincate (2-) anions (Fig. 4.1.4). The formed Zn-O bonds are dative bonds indicated as arrows pointing from the O to the Zn.
The Lewis acid-base concept can even be used to explain bonding in ionic crystals. In this case the anion would be the donor and the cation the acceptor.
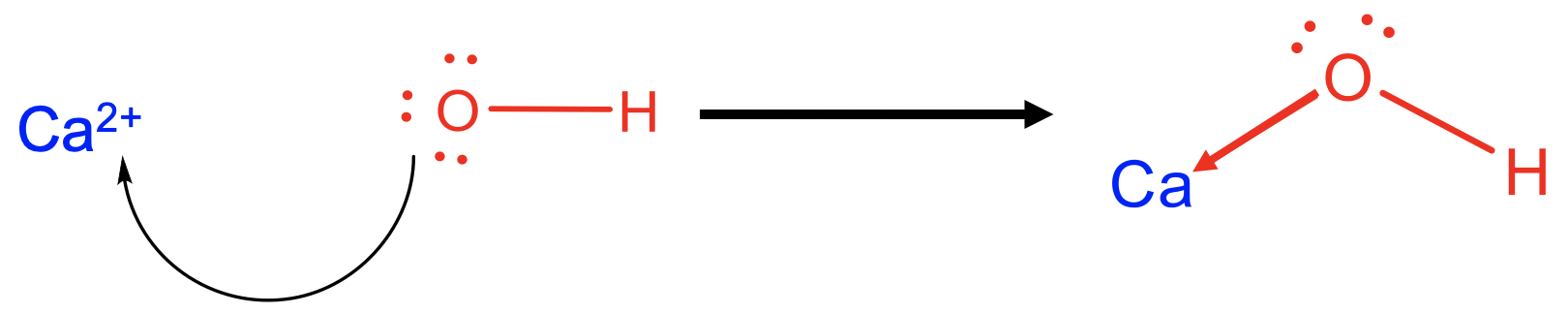
For example Ca2+ can be viewed as a Lewis acid, and OH- as a Lewis base in a reaction that forms calcium hydroxide Ca(OH)2 as the Lewis acid-base adduct. The bonds between Ca2+ and OH- would be viewed as a dative bonds indicated as arrows pointing from O to Ca. In this case the ionic bonds would be interpreted as highly polar dative bonds. You may want to remember in this context that completely ionic bonds are not possible in general, and there must also be some degree of covalency due to the finite electronegativity difference between two elements.
MO theory and Lewis Bases
MO theory is a theory designed to explain covalent bonding. Because dative bonds are covalent bonds, MO theory should be able to explain dative bonds. So how can Lewis acids and bases and their reactions be viewed from an MO theory perspective? MO theory states that a molecule is a Lewis base when its HOMO is approximately non-bonding (Fig. 4.1.6).
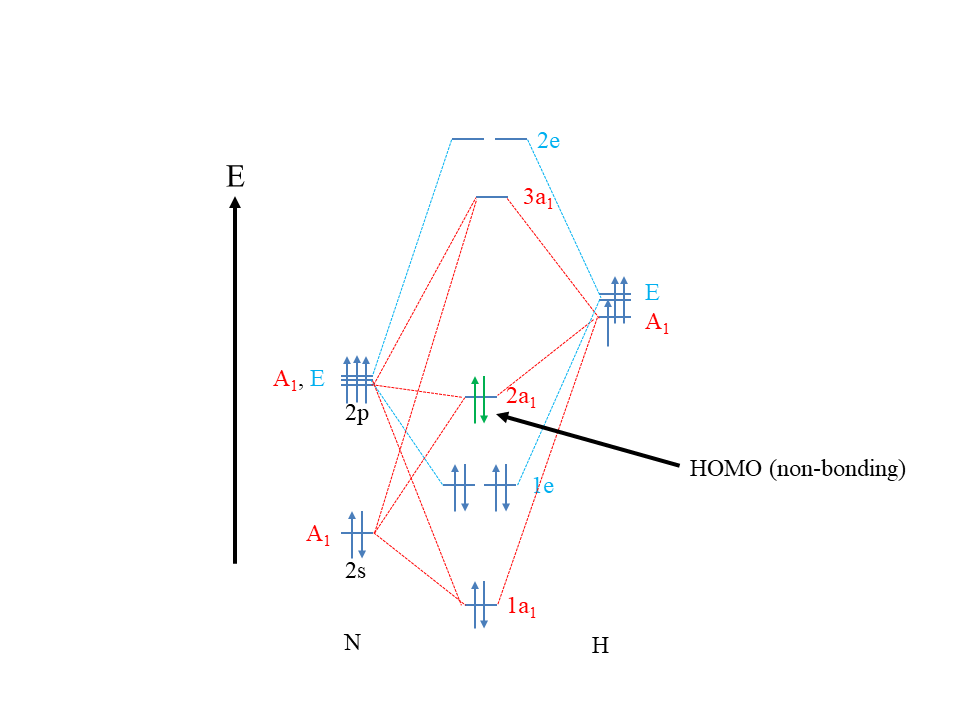
This explains, for example, that NH3 is a Lewis base. Remember, its HOMO, the 2a1 orbital, is approximately non-bonding. Why does an approximately non-bonding HOMO make a molecule a Lewis base? Firstly, because the HOMO electrons are the highest energy electrons and thus the most reactive electrons, they get donated preferentially over all other electrons. The non-bonding nature of the HOMO is ideal, because if the HOMO was anti-bonding, the electrons would be so reactive, so that they would likely get completely transferred to the reaction partner. In this case we would not have a Lewis acid-base reaction, but a redox reaction. We would not form a covalent bond, but an ionic bond. If the HOMO was bonding, then the energy of the electrons would be too unreactive, simply no reaction would likely be observed.
MO theory and Lewis Acids
So what is then a Lewis acid according to MO theory? A Lewis acid, from the MO theory perspective, is a molecule that has an approximately non-bonding lowest unoccupied molecular orbital. The orbital needs to unoccupied, otherwise no electrons could be donated into it. For energy minimization arguments, electrons would be donated into the unoccupied orbital that has the lowest energy, which is the LUMO. So why is it ideal if the LUMO is non-bonding? If it was anti-bonding, its energy would likely be too high, and the donor would be unable to donate its electrons. There would not be a stabilization of electrons due to the high energy of the LUMO. The molecule would remain unreactive. If the LUMO was a bonding orbital, then its energy would likely be so low so that the electrons would likely be completely transferred to the Lewis acid. In this case we would not form a dative bond but an ionic bond via a redox reaction.
Bonding in Lewis Acid Base Adducts
The greatest covalent interaction between two orbitals it is achieved when both orbitals have the same energy. We learned this previously in Chapter 3 when we discussed the energy criterion. The same holds for dative bonds. The more similar the energies of the donor HOMO and the LUMO acceptor, the greater the covalent interaction. Ideally, the energies are exactly same. In this case we form a perfect covalent bond with the electrons equally shared between the donor and the acceptor. We can graphically illustrate this in an MO diagram the following way (Fig. 4.1.9).
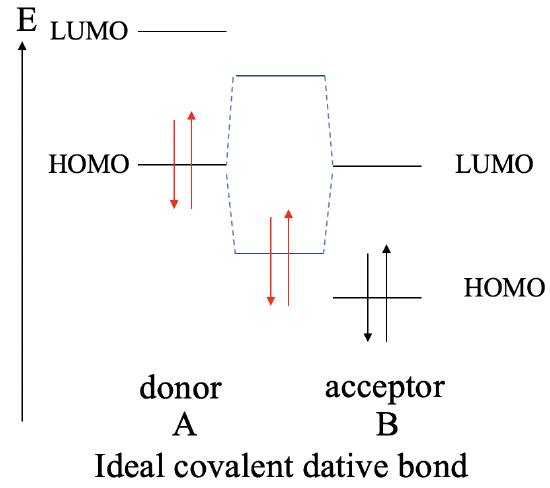
Let us assume a molecule A that acts as a donor and a molecule B that acts as an acceptor. The donor A has a HOMO and a LUMO that have a certain energy. The acceptor molecule B also has a HOMO and a LUMO with the LUMO having the same energy as the HOMO of A. The molecule B will also have HOMO which is energetically below that LUMO. Because their identical energies the combination of the HOMO of A with the LUMO of B leads to a bonding MO and an anti-bonding MO with equally shared electron density. Both MOs have equidistant energy from the HOMO of A and the LUMO of B. The electrons coming from the HOMO of A will be in the bonding MO. They will be equally shared, and the dative bond is an ideal covalent bond.
The exact match of the HOMO and LUMO energies of the donor and acceptor is rarely achieved. Let us consider a number of scenarios in which these energies are not the same, and what consequences this has for the dative bond.
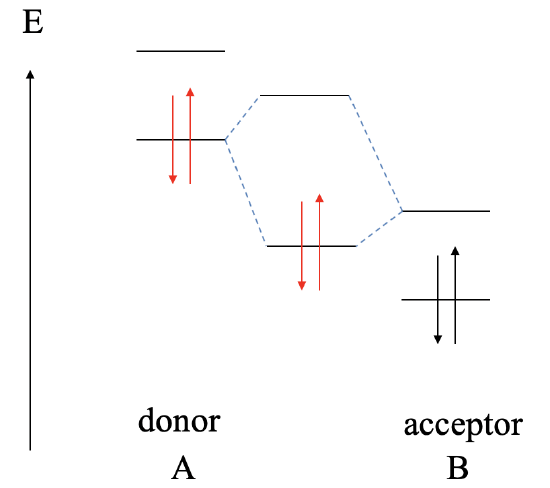
Let us assume next, that the LUMO of the acceptor B is somewhat lower than the HOMO of donor A. In this case, we can still form a bonding MO and an anti-bonding MO due to covalent interaction between the HOMO of A and the LUMO of B. However, now the bonding MO will be localized primarily at B, and the anti-bonding MO will be localized mostly at A. The electrons from the HOMO of A will be in the bonding MO, and thus the bonding electrons in the dative bond will be localized mostly at B. This means that the dative bond is polar, and polarized toward the acceptor.
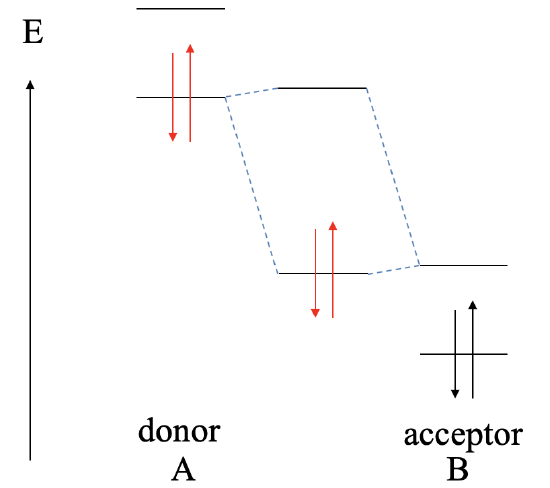
Let us consider next, what the bonding will be if the LUMO of the acceptor is much lower than the HOMO of the donor (Fig. 4.1.11). In this case, there is still the possibility to form a bonding MO and an anti-bonding MO, but the bonding MO will be localized practically exclusively at the acceptor, and the anti-bonding MO will be localized practically completely ar the donor. The electrons from the donor will be in the bonding MO, but because the bonding MO is located almost entirely at the acceptor, the electrons are transferred completely from A to B in a redox reaction, and the bonding will be ionic, and we have an ionic compound AB made of A2+ cations and B2- anions. This reaction would no longer be considered a Lewis acid-base reaction, and the reaction product will no longer be considered a Lewis acid-base adduct.
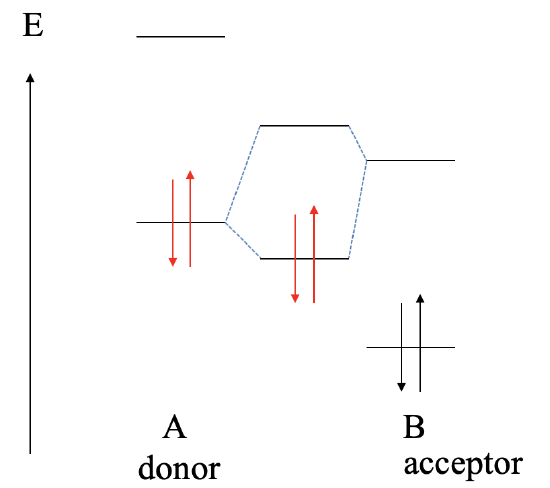
The next possibility to consider is that the LUMO of B is higher in energy than the HOMO of A (Fig. 4.1.12). In this case the bonding MO will be localized primarily at the donor A and the electrons in the dative bond will be predominantly at A. We have a polar, dative bond which is polarized toward A.
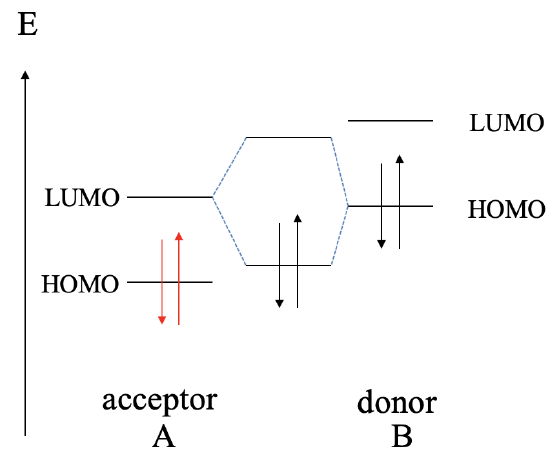
Next, let us raise the energy of the HOMO and the LUMO of B even higher. This leads to the fact that now the HOMO of B is energetically closer to the LUMO of A, compared to the energy difference between the HOMO of A and the LUMO of B. This results in the fact that the interaction will be mostly between the HOMO of B and and the LUMO of A. As a consequence, B will act now as the donor, and A will act as the acceptor. The dative bond may be polarized toward A or B, or not be polar at all depending on the relative energy of the HOMO of B and the LUMO of A.
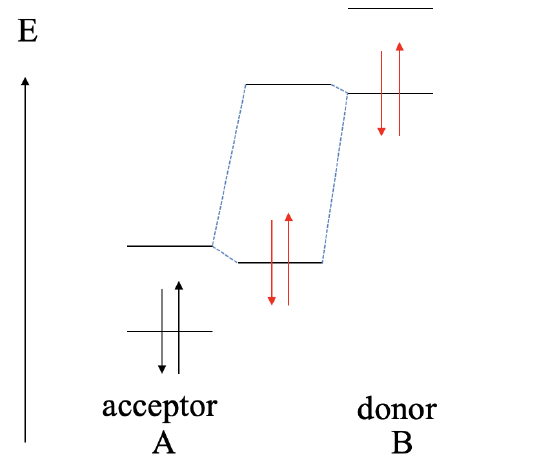
If we raise the orbital energies of B even further, and the HOMO of B is much higher than the HOMO of A, then we will get a redox reaction. B gets oxidized, and A gets reduced. B2+ cations and A2- anions will form an ionic compound of the composition AB.
Examples
Generally spoken, the relative HOMO and LUMO energies of reaction partners decide if a Lewis acid-base, or a redox reaction takes place, and what the polarity of the bond is. Here are a few examples that will illustrate our general considerations (Fig. 4.1.15).
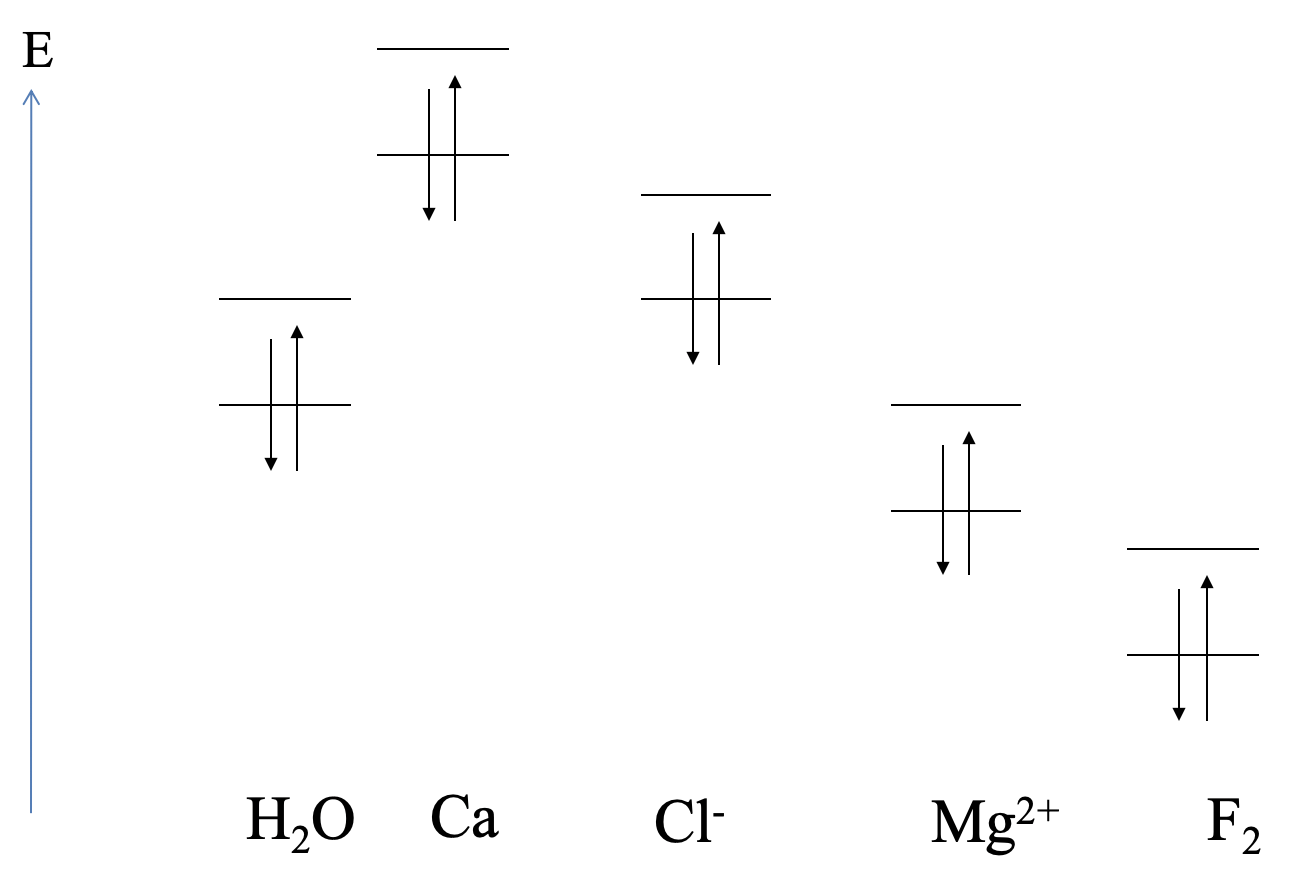
The relative orbital energies of the highest occupied atomic orbitals and the lowest unoccupied atomic orbitals of calcium, and the HOMO and LUMO energies of H2O are shown (Fig. 4.1.15). Can we predict the type of reaction? We can see that the highest occupied Ca orbital has a much higher energy than the LUMO of water. We would therefore expect that Ca gets oxidized, and water gets reduced. An ionic compound would be expected. This is what actually happens in experiment. The reaction of water and calcium yields calcium hydroxide and hydrogen gas.
Next, let us consider a possible reaction between water and chloride. We can see that the HOMO of Cl- is similar in energy compared to the LUMO of H2O. We would therefore expect a Lewis acid-base interaction with Cl- as the donor and H2O as the acceptor. Such an interaction indeed occurs in aqueous solutions containing Cl- in the form of weak hydrogen bonding between Cl- and H2O.
Next, let us consider the interactions between Mg2+ and H2O. In this case the LUMO of Mg2+ has about the energy of the HOMO of the water molecule. We would therefore expect that the water molecule acts as the donor and the Mg2+ acts as the acceptor. Indeed, Mg2+ forms a hexaaqua complex with water, which has the composition Mg(H2O)62+. The bonding should be very little polar.
Lastly, what are the interactions between F2 and H2O? The HOMO of H2O is much higher than the LUMO of F2. We would therefore expect a redox reaction in which F2 is reduced, and H2O is oxidized. In reality F2 can oxidize H2O to form OF2 and HF.
From the above examples it becomes also clear that we cannot necessarily predict the strength of the Lewis-acid base interactions. For example, the hydrogen bonding between H2O and Cl- is much weaker than the dative bonds between H2O and Mg2+. Other factors such as orbital overlap also need to be taken into consideration to make statements about the strength of the interactions.
Dr. Kai Landskron (Lehigh University). If you like this textbook, please consider to make a donation to support the author's research at Lehigh University: Click Here to Donate.


