4.4: Atomic Theory
- Page ID
- 218484
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
Learning Objectives
- State the modern atomic theory.
- Learn how atoms are constructed.
- Describe the locations, charges, and masses of the three main subatomic particles.
- Determine the number of protons and electrons in an atom.
- Define atomic mass unit (amu).
The smallest piece of an element that maintains the identity of that element is called an atom. Individual atoms are extremely small. It would take about fifty million atoms in a row to make a line that is 1 cm long. The period at the end of a printed sentence has several million atoms in it. Atoms are so small that it is difficult to believe that all matter is made from atoms-but it is.
The concept that atoms play a fundamental role in chemistry is formalized by the modern atomic theory, first stated by John Dalton, an English scientist, in 1808. It consists of three parts:
- All matter is composed of atoms.
- Atoms of the same element are the same; atoms of different elements are different.
- Atoms combine in whole-number ratios to form compounds.
These concepts form the basis of chemistry. Although the word atom comes from a Greek word that means "indivisible," we understand now that atoms themselves are composed of smaller parts called subatomic particles. The first part to be discovered was the electron, a tiny subatomic particle with a negative charge. It is often represented as e−, with the right superscript showing the negative charge. Later, two larger particles were discovered. The proton, a subatomic particle with a positive charge. is a more massive (but still tiny) subatomic particle with a positive charge, represented as p. The neutron is a subatomic particle with about the same mass as a proton but no charge. It is represented as either n. We now know that all atoms of all elements are composed of electrons, protons, and (with one exception) neutrons. Table \(\PageIndex{1}\) summarizes the properties of these three subatomic particles.
Even though electrons, protons, and neutrons are all types of subatomic particles, they are not all the same size. When you compare the masses of electrons, protons, and neutrons, what you find is that electrons have an extremely small mass, compared to either protons or neutrons. On the other hand, the masses of protons and neutrons are fairly similar, although technically, the mass of a neutron is slightly larger than the mass of a proton. Because protons and neutrons are so much more massive than electrons, almost all of the mass of any atom comes from the nucleus, which contains all of the neutrons and protons.
| Particle | Symbol | Mass (amu) | Relative Mass (proton = 1) | Relative Charge | Location |
|---|---|---|---|---|---|
| proton | p | 1 | 1 | +1 | inside the nucleus |
| electron | e− | 5.45 × 10−4 | 0.00055 | −1 | outside nucleus |
| neutron | n | 1 | 1 | 0 | inside the nucleus |
The third column shows the masses of the three subatomic particles in "atomic mass units." An atomic mass unit (amu) is defined as one-twelfth the mass of a carbon-12 atom. Atomic mass units (amu) are useful, because, as you can see, the mass of a proton and the mass of a neutron are almost exactly 11 in this unit system.
How are these particles arranged in atoms? They are not arranged at random. Experiments by Ernest Rutherford in England in the 1910s pointed to a nuclear model with atoms that has the protons and neutrons in a central nucleus with the electrons in orbit about the nucleus. The relatively massive protons and neutrons are collected in the center of an atom, in a region called the nucleus of the atom (plural nuclei). The electrons are outside the nucleus and spend their time orbiting in space about the nucleus. (Figure \(\PageIndex{1}\)).
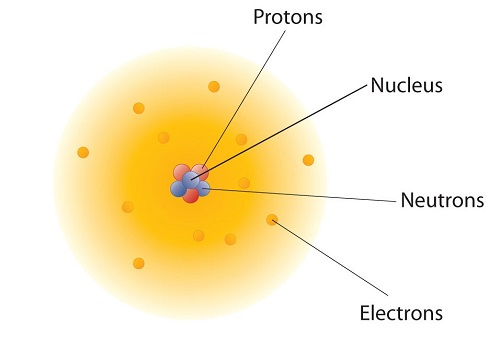
The modern atomic theory states that atoms of one element are the same, while atoms of different elements are different. What makes atoms of different elements different? The fundamental characteristic that all atoms of the same element share is the number of protons. All atoms of hydrogen have one and only one proton in the nucleus; all atoms of iron have 26 protons in the nucleus. This number of protons is so important to the identity of an atom that it is called the atomic number. The number of protons in an atom is the atomic number of the element (Z). Thus, hydrogen has an atomic number of 1, while iron has an atomic number of 26. Each element has its own characteristic atomic number.
Atoms of the same element can have different numbers of neutrons, however. Atoms of the same element (i.e., atoms with the same number of protons) with different numbers of neutrons are called isotopes. The sum of the number of protons and neutrons in the nucleus is called the mass number of the isotope. We will discuss isotopes and their symbols more in section 4.5 of this chapter.
Neutral atoms have the same number of electrons as they have protons, so their overall charge is zero. Negative and positive charges of equal magnitude cancel each other out. This means that the negative charge on an electron perfectly balances the positive charge on the proton. In other words, a neutral atom must have exactly one electron for every proton. If a neutral atom has 1 proton, it must have 1 electron. If a neutral atom has 2 protons, it must have 2 electrons. If a neutral atom has 10 protons, it must have 10 electrons. You get the idea. In order to be neutral, an atom must have the same number of electrons and protons. If the number of electrons and protons are not equal, the atom is called an ion. Ions and ionic compounds will be discussed in detail in section 4.10 and chapter 5.
Example \(\PageIndex{1}\):
- The most common carbon atoms have six protons and six neutrons in their nuclei. What are the atomic number and the mass number of these carbon atoms?
- An isotope of uranium has an atomic number of 92 and a mass number of 235. What are the number of protons and neutrons in the nucleus of this atom?
Solution
- If a carbon atom has six protons in its nucleus, its atomic number is 6. If it also has six neutrons in the nucleus, then the mass number is 6 + 6, or 12.
- If the atomic number of uranium is 92, then that is the number of protons in the nucleus. Because the mass number is 235, then the number of neutrons in the nucleus is 235 − 92, or 143.
Exercise \(\PageIndex{1}\)
The number of protons in the nucleus of a tin atom is 50, while the number of neutrons in the nucleus is 68. What are the atomic number and the mass number of this isotope?
- Answer
-
Atomic number = 50, mass number = 118
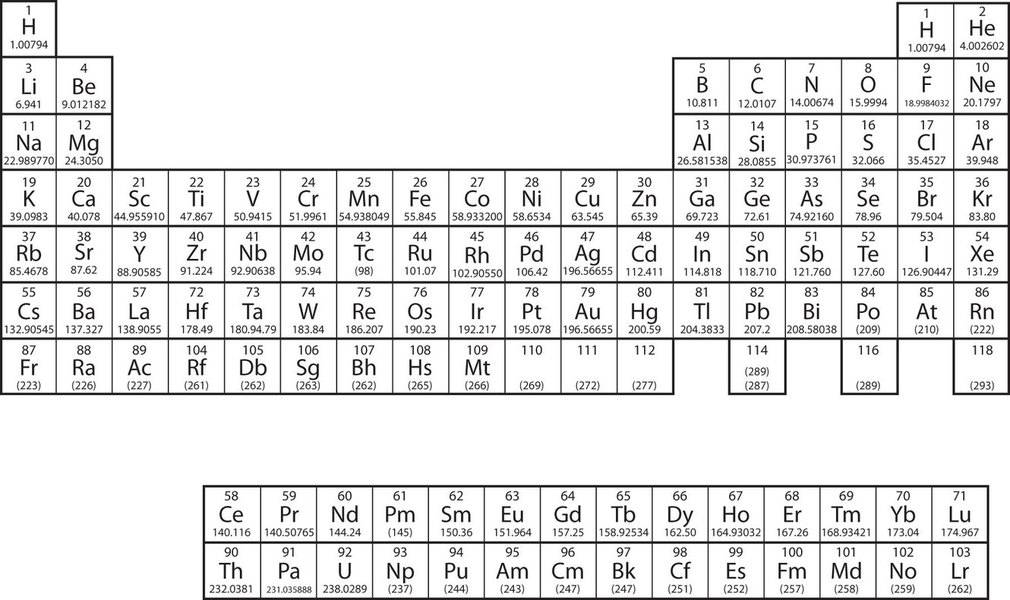
When referring to an atom, we simply use the element's name: the term sodium refers to the element as well as an atom of sodium. But it can be unwieldy to use the name of elements all the time, and therefore scientists use the chemical symbols discussed in section 1.2 and 4.2.The elements are grouped together in a special chart called the periodic table of all the elements. A simple periodic table is shown in Figure \(\PageIndex{2}\), while one may view a more extensive periodic table. The elements on the periodic table are listed in order of ascending atomic number. We will learn more about how the periodic table is organized as we move through the semester. One immediate use of the periodic table helps us identify metals and nonmetals. Nonmetals are in the upper right corner of the periodic table, on one side of the heavy line splitting the right-hand part of the chart. All other elements are metals.