4.5: Defining Isotopes
- Last updated
- Save as PDF
- Page ID
- 218486
Learning Objectives
- Define and differentiate between the atomic number and the mass number of an element.
- Explain how isotopes differ from one another.
Now that we know how atoms are generally constructed, what do atoms of any particular element look like? How many protons, neutrons, and electrons are in a specific kind of atom? First, if an atom is electrically neutral overall, then the number of protons equals the number of electrons. Because these particles have the same but opposite charges, equal numbers cancel out, producing a neutral atom.
Atomic Number
In the 1910s, experiments with x-rays led to this useful conclusion: the magnitude of the positive charge in the nucleus of every atom of a particular element is the same. In other words, all atoms of the same element have the same number of protons. Furthermore, different elements have a different number of protons in their nuclei, so the number of protons in the nucleus of an atom is characteristic of a particular element. This discovery was so important to our understanding of atoms that the number of protons in the nucleus of an atom is called the atomic number (Z).
For example, hydrogen has the atomic number 1; all hydrogen atoms have 1 proton in their nuclei. Helium has the atomic number 2; all helium atoms have 2 protons in their nuclei. There is no such thing as a hydrogen atom with 2 protons in its nucleus; a nucleus with 2 protons would be a helium atom. The atomic number defines an element. Table \(\PageIndex{1}\) lists some common elements and their atomic numbers. Based on its atomic number, you can determine the number of protons in the nucleus of an atom. The largest atoms have over 100 protons in their nuclei.
| Element | Atomic Number | Element | Atomic Nmbers |
|---|---|---|---|
| aluminum (Al) | 13 | magnesium (Mg) | 12 |
| americium (Am) | 95 | manganese (Mn) | 25 |
| argon (Ar) | 18 | mercury (Hg) | 80 |
| barium (Ba) | 56 | neon (Ne) | 10 |
| beryllium (Be) | 4 | nickel (Ni) | 28 |
| bromine (Br) | 35 | nitrogen (N) | 7 |
| calcium (Ca) | 20 | oxygen (O) | 8 |
| carbon (C) | 6 | phosphorus (P) | 15 |
| chlorine (Cl) | 17 | platinum (Pt) | 78 |
| chromium (Cr) | 24 | potassium (K) | 19 |
| cesium (Cs) | 55 | radon (Rn) | 86 |
| fluorine (F) | 9 | silver (Ag) | 47 |
| gallium (Ga) | 31 | sodium (Na) | 11 |
| gold (Au) | 79 | strontium (Sr) | 38 |
| helium (He) | 2 | sulfur (S) | 16 |
| hydrogen (H) | 1 | titanium (Ti) | 22 |
| iron (Fe) | 26 | tungsten (W) | 74 |
| iodine (I) | 53 | uranium (U) | 92 |
| lead (Pb) | 82 | zinc (Zn) | 30 |
| lithium (Li) | 3 | zirconium (Zr) | 40 |
Example \(\PageIndex{1}\)
What is the number of protons in the nucleus of each element?
- aluminum
- iron
- carbon
- Answer a
-
According to Table 2.4.1, aluminum has an atomic number of 13. Therefore, every aluminum atom has 13 protons in its nucleus.
- Answer b
-
Iron has an atomic number of 26. Therefore, every iron atom has 26 protons in its nucleus.
- Answer c
-
Carbon has an atomic number of 6. Therefore, every carbon atom has 6 protons in its nucleus.
Exercise \(\PageIndex{1}\)
What is the number of protons in the nucleus of each element? Use Table 2.4.1.
- sodium
- oxygen
- chlorine
- Answer a
-
Sodium has 11 protons in its nucleus.
- Answer b
-
Oxygen has 8 protons in its nucleus.
- Answer c
-
Chlorine has 17 protons in its nucleus
How many electrons are in an atom? Previously we said that for an electrically neutral atom, the number of electrons equals the number of protons, so the total opposite charges cancel. Thus, the atomic number of an element also gives the number of electrons in an atom of that element. (Later we will find that some elements may gain or lose electrons from their atoms, so those atoms will no longer be electrically neutral. Thus we will need a way to differentiate the number of electrons for those elements.)
Example \(\PageIndex{2}\)
How many electrons are present in the atoms of each element?
- sulfur
- tungsten
- argon
- Answer a
-
The atomic number of sulfur is 16. Therefore, in a neutral atom of sulfur, there are 16 electrons.
- Answer b
-
The atomic number of tungsten is 74. Therefore, in a neutral atom of tungsten, there are 74 electrons.
- Answer c
-
The atomic number of argon is 18. Therefore, in a neutral atom of argon, there are 18 electrons.
Exercise \(\PageIndex{2}\)
How many electrons are present in the atoms of each element?
- magnesium
- potassium
- iodine
- Answer a
-
Mg has 12 electrons.
- Answer b
-
K has 19 electrons.
- Answer c
-
I has 53 electrons.
Isotopes
How many neutrons are in atoms of a particular element? At first it was thought that the number of neutrons in a nucleus was also characteristic of an element. However, it was found that atoms of the same element can have different numbers of neutrons. Atoms of the same element (i.e., same atomic number, Z) that have different numbers of neutrons are called isotopes. For example, 99% of the carbon atoms on Earth have 6 neutrons and 6 protons in their nuclei; about 1% of the carbon atoms have 7 neutrons in their nuclei. Naturally occurring carbon on Earth, therefore, is actually a mixture of isotopes, albeit a mixture that is 99% carbon with 6 neutrons in each nucleus.
An important series of isotopes is found with hydrogen atoms. Most hydrogen atoms have a nucleus with only a single proton. About 1 in 10,000 hydrogen nuclei, however, also has a neutron; this particular isotope is called deuterium. An extremely rare hydrogen isotope, tritium, has 1 proton and 2 neutrons in its nucleus. Figure \(\PageIndex{1}\) compares the three isotopes of hydrogen.
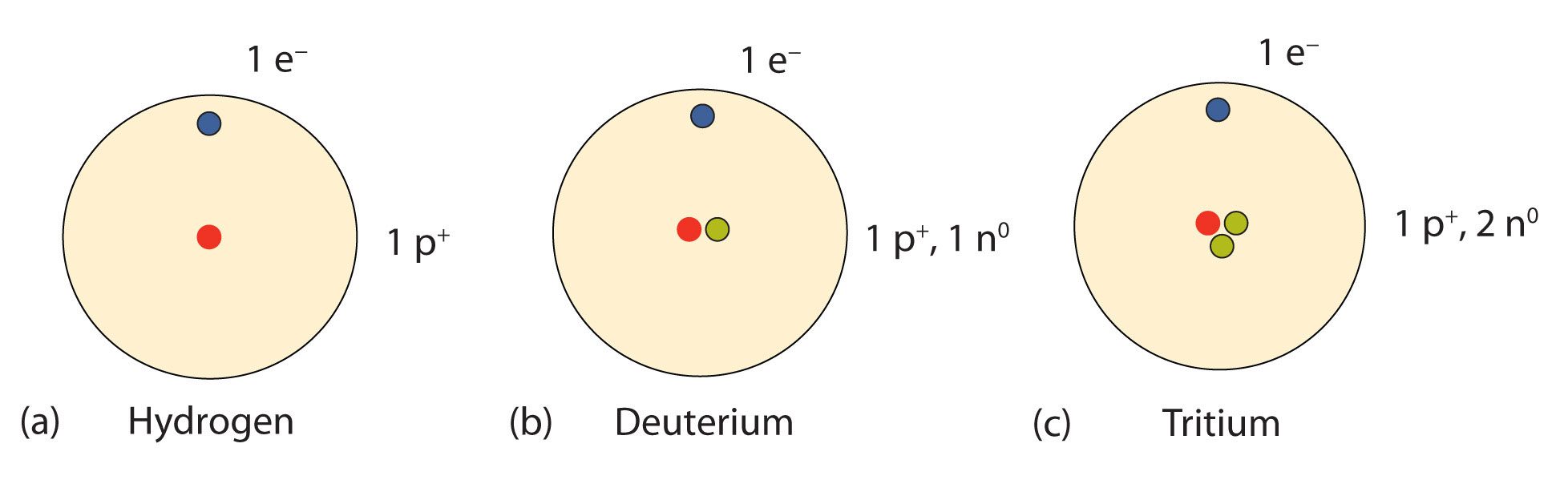
The discovery of isotopes required a minor change in Dalton’s atomic theory. Dalton thought that all atoms of the same element were exactly the same.
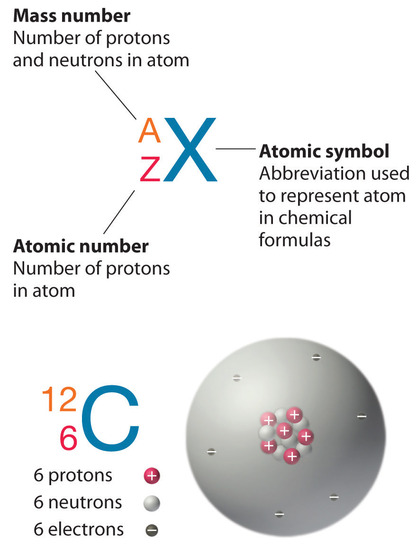
Most elements exist as mixtures of isotopes. In fact, there are currently over 3,500 isotopes known for all the elements. When scientists discuss individual isotopes, they need an efficient way to specify the number of neutrons in any particular nucleus. The mass number (A) of an atom is the sum of the numbers of protons and neutrons in the nucleus. Given the mass number for a nucleus (and knowing the atomic number of that particular atom), you can determine the number of neutrons by subtracting the atomic number from the mass number.
A simple way of indicating the mass number of a particular isotope is to list it as a superscript on the left side of an element’s symbol. Atomic numbers are often listed as a subscript on the left side of an element’s symbol. Thus, we might see
\[\mathrm{^{mass\: number\xrightarrow{\hspace{45px}} 56}_{atomic\: number \xrightarrow{\hspace{35px}} 26}Fe} \label{Eq1} \]
which indicates a particular isotope of iron. The 26 is the atomic number (which is the same for all iron atoms), while the 56 is the mass number of the isotope. To determine the number of neutrons in this isotope, we subtract 26 from 56: 56 − 26 = 30, so there are 30 neutrons in this atom.
Example \(\PageIndex{3}\)
How many protons and neutrons are in each atom?
- \(\mathrm{^{35}_{17}Cl}\)
- \(\mathrm{^{127}_{53}I}\)
- Answer a
-
In \(\mathrm{^{35}_{17}Cl}\) there are 17 protons, and 35 − 17 = 18 neutrons in each nucleus.
- Answer b
-
In \(\mathrm{^{127}_{53}I}\) there are 53 protons, and 127 − 53 = 74 neutrons in each nucleus.
Exercise \(\PageIndex{3}\)
How many protons and neutrons are in each atom?
- \(\mathrm{^{197}_{79}Au}\)
- \(\mathrm{^{23}_{11}Na}\)
- Answer a
-
In \(\mathrm{^{197}_{79}Au}\) there are 79 protons, and 197 − 79 = 118 neutrons in each nucleus.
- Answer b
-
In \(\mathrm{^{23}_{11}Na}\) there are 11 protons, and 23 − 11 = 12 neutrons in each nucleus.
It is not absolutely necessary to indicate the atomic number as a subscript because each element has its own unique atomic number. Many isotopes are indicated with a superscript only, such as 13C or 235U. You may also see isotopes represented in print as, for example, carbon-13 or uranium-235.
Summary
The atom consists of discrete particles that govern its chemical and physical behavior. Each atom of an element contains the same number of protons, which is the atomic number (Z). Neutral atoms have the same number of electrons and protons. Atoms of an element that contain different numbers of neutrons are called isotopes. Each isotope of a given element has the same atomic number but a different mass number (A), which is the sum of the numbers of protons and neutrons.
Almost all of the mass of an atom is from the total protons and neutrons contained within a tiny (and therefore very dense) nucleus. The majority of the volume of an atom is the surrounding space in which the electrons reside. A representation of a carbon-12 atom is shown below in Figure \(\PageIndex{2}\).
Concept Review Exercises
- Why is the atomic number so important to the identity of an atom?
- What is the relationship between the number of protons and the number of electrons in an atom?
- How do isotopes of an element differ from each other?
- What is the mass number of an element?
Answers
- The atomic number defines the identity of an element. It describes the number of protons in the nucleus.
- In an electrically neutral atom, the number of protons equals the number of electrons.
- Isotopes of an element have the same number of protons but have different numbers of neutrons in their nuclei.
- The mass number is the sum of the numbers of protons and neutrons in the nucleus of an atom.
Key Takeaways
- Each element is identified by its atomic number. The atomic number provides the element's location on the periodic table
- The isotopes of an element have different masses and are identified by their mass numbers.

