9.1: Question 9.E.1 PASS - butane Newman projection, bond rotation energy diagram
- Page ID
- 452336
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Consider butane’s C2—C3 bond:
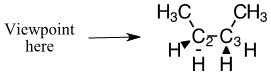
a) Draw the Newman Projection as shown above.
b) Draw the energy profile when the back carbon (C3) is rotated in 60° clockwise intervals.
c) Label each conformation.
- Answer
-
a) Newman projection:
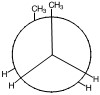
b) & c)

See LibreText 9.1 Rotation about single bonds - conformations
- Strategy Map
-
Step Hint 1. Using the viewpoint given in the question, sketch the Newman Projection of the compound. 2. Sketch each phase as the back carbon (C3) rotates clockwise. Recall how to draw Newman projections (see LibreText section 9.1)
3. Create a graph depicting the energy change due to the stability of each position. The closer the atoms are, the more interactions there will be. The further they are, the more stable the compound. 4. Label each position with its corresponding name. The possible names include Staggered Gauche, Eclipse, Staggered Anti and Full eclipse (not in any order).
- Solution
-
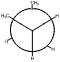
a)

The exterior circle represents the forward carbon (C2) and the interior center represents the back carbon (C3).
b) & c)
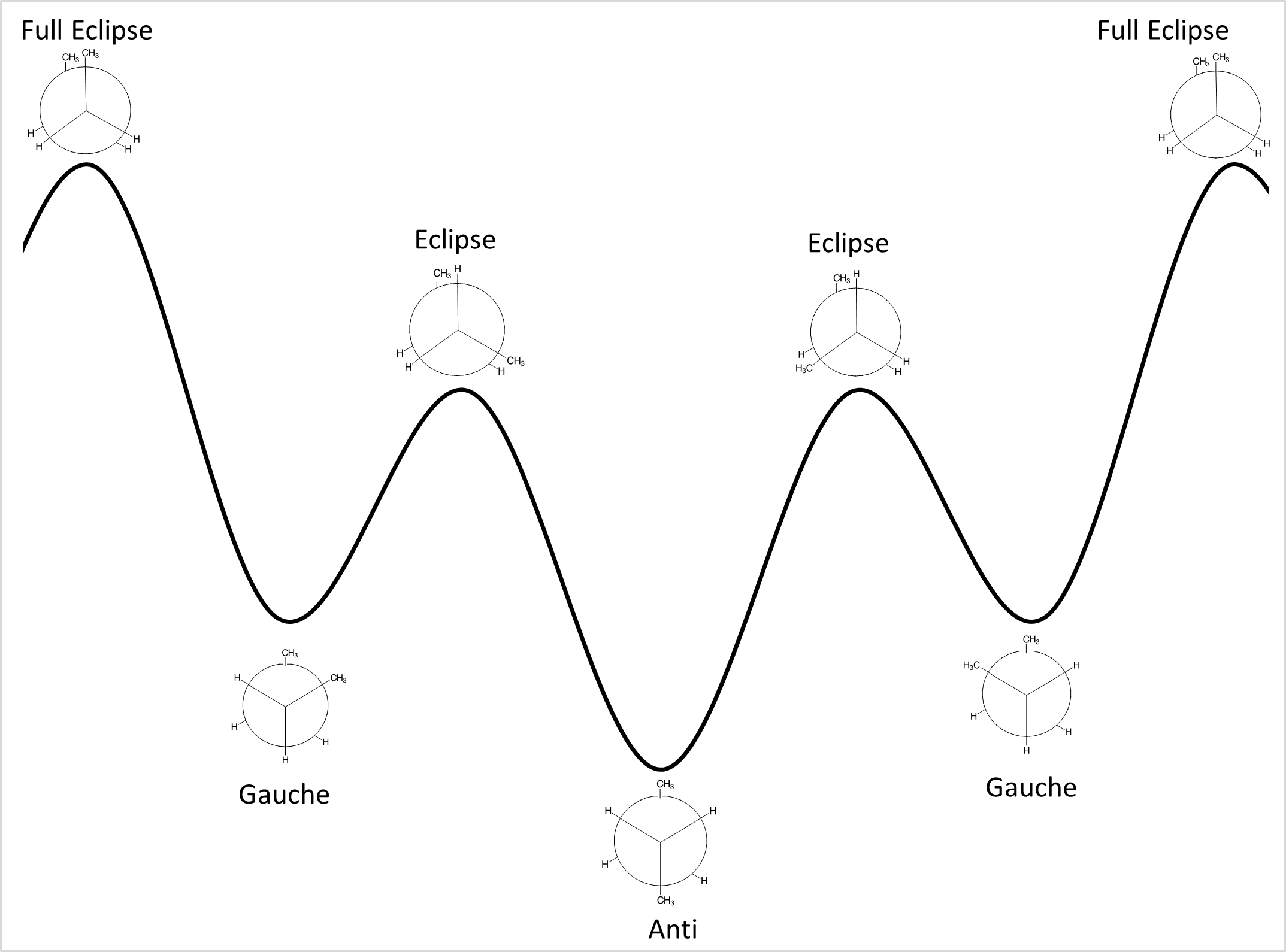
(Least stable) Full eclipse < Eclipse < Staggered Gauche < Staggered Anti (Most stable)
- Guided Solution
-
Download Guided Solution as a pdf
Guided Solution Hint This is a theory type problem that tests your knowledge on the different conformations of a compound and the stability of each conformation. See LibreText 9.1 Rotation about single bonds - conformations Question:
Consider butane’s C2—C3 bond:

a) Draw the Newman Projection as shown above.
b) Draw the energy profile when the back carbon (C3) is rotated in 60° clockwise intervals.
c) Label each conformation.
How to sketch a Newman Projection:
1. Identify your viewpoint (which bond are you looking at).
2. Draw the skeleton shape of your compound. A line represents the back carbon, and a circle represents the front carbon.
3. Draw the groups attached to the front carbon.
4. Draw the attached groups to the back carbon.
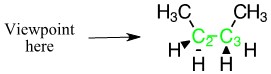
Looking down the carbon-carbon bond. Carbon two in the front, carbon three in the back.

The back carbon is represented in green, and the front carbon is represented in black.
 With the substituent groups on the front carbon
With the substituent groups on the front carbon With the substituent groups on the back carbon.
With the substituent groups on the back carbon.How much is a 60-degree rotation?
There are 360 degrees in a circle, if the bond rotates 60 degrees, it is rotating 1/6 of a circle.

What impacts the stability of a compounds conformation? Due to interactions between different atoms, certain positions will have higher stability than others. Certain positions will cause more atoms to be in closer contact and they must overcome more repulsions. This requires energy and therefore causes the compound to be less stable. Recall the name of each conformation.
The possible names include Staggered Gauche, Eclipse, Staggered Anti and Full eclipse (not in any order).
Staggered Gauche

Eclipse


Staggered Anti

Full eclipse

Complete Solution:
a) Newman Projection:

This figure depicts the compound butane down the carbon-carbon bond. The exterior circle represents the forward carbon (C2) and the interior center represents the back carbon (C3) The back carbon will rotate at 60° angles.
Step
b) & c)
Energy profile with labeled conformations:

The diagram shows the seven stages of a butane compounds rotation as well as the different levels of stability for each.
(Least stable) Full eclipse < Eclipse < Staggered Gauche < Staggered Anti (Most stable)
The starting phase is Full eclipse, with both of the methane groups side by side. This requires the most energy and therefore is the least stable position.
After the first 60° rotation, the position of the compound is called staggered gauche. The methane group on the back carbon (C3) is now to the left of the methane group on the front carbon (C2). This position is significantly more stable.
After the next 60° rotation, the position of the compound is called eclipse. The methane on the back carbon (C3) is now overlapping with the hydrogen on the front carbon (C2). This position is slightly less stable than the last.
After the next 60° rotation, the position of the compound is called Staggered Anti. The methane group on the back carbon (C3) is now in the opposite position to the methane group on the front carbon (C2). This position is the most stable.
After the next 60° rotation, the position of the compound is called eclipse. The methane on the back carbon (C3) is now overlapping with the hydrogen on the front carbon (C2). This position is slightly less stable than the last.
After the next 60° rotation, the position of the compound is called staggered gauche. The methane group on the back carbon (C3) is now to the right of the methane group on the front carbon (C2). This position is more stable than the previous.
After one more 60° rotation, the compound is back to the first position of full eclipse.
Molecular model of the Newman Projection in Full Eclipse:

Molecular model of the Newman projection in Staggered Gauche:

Molecular model of the Newman projection in Eclipse:

Molecular model of the Newman projection in Staggered Anti:

Molecular model of the Newman projection in eclipse:

Molecular model of the Newman projection in Staggered Gauche:

Check your work!
Make sure you are working from the point of view in the question. Working with a model is really helpful.
Why does this answer make chemical sense?
Molecules are constantly moving around in 3D space. Single bonds will twist and rotate. Due to interactions between different atoms, certain positions will have higher stability than others. We can analyze the stability of these positions by looking at the atoms in close contact.
See LibreText 9.1 Rotation about single bonds - conformations
(Original question authored by Lindsay Blackstock, shared under a CC BY-NC-SA 4.0 license)


