9.2: Question 9.E.2 PASS - cycloalkane chair, axial vs. equatorial, most stable
- Page ID
- 452337
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Consider: Trans 1,2-dibromocyclohexane
- Draw the simple line structure.
- Draw the two chair confirmations, numbering the carbons and labelling the positions as axial/equatorial.
- Identify which is the more stable structure.
- Answer
-
Simple Line Structure:

Chair conformations:
axial conformation

equatorial conformation
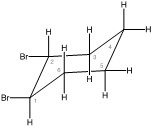
The equatorial conformation is the most stable.
- Strategy Map
-
Step Hint 1. Use the provided molecule name to draw its simple line structure. Recall drawing chemical structures, line formula (see LibreText section 7.2) 2. Draw the templates of the two possible conformations. See LibreText 9.4 Cyclohexane - A Strain-Free Cycloalkane (How to draw Axial and Equatorial bonds)
3. Number the carbons one through six. 4. Label the axial and equatorial positions. 5. Convert your simple line structure into your two templates.
6. Compare the two conformations and identify which would be the most stable. The most stable conformation is the one that has the least number of interactions between substituent atoms. The larger, more electronegative atoms are favoured in the equatorial positions as they will be impacted by less interactions with surrounding atoms.
- Solution
-
1. Simple Line Structure:

This molecule is trans meaning one bromine is dashed back and one is wedged forward. These bromines are on a six-carbon ring and are attached to carbons one and two.
Chair conformations:
axial

Both bromines are in axial positions.
equatorial

Both bromines are in equatorial positions.
The equatorial conformation is the most stable. It has the least interactions between the bromine groups and surrounding atoms.
- Guided Solution
-
Download Guided Solution as a pdf
Guided Solution Hint This is a theory type problem that tests your understanding of cycloalkane naming and chair conformations. See LibreText 9.4 Cyclohexane - A Strain-Free Cycloalkane and see LibreText 9.5 Substituted Cyclohexanes Consider: Trans 1,2-dibromocyclohexane
Draw the simple line structure. Draw the two chair confirmations, numbering the carbons and labelling the positions as axial/equitorial. Identify which is the more stable structure.
Recall how to sketch a line structure:
- Identify if the structure is Cis or Trans.
- Determine if the parent chain is in the form of a chain or ring. (This will be at the end of the name).
- Identify substituent atoms and which carbons they are connected to.
Trans 1,2-dibromocyclohexane
This is a cyclohexane, which tells us it is a ring structure.
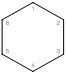
The numbers tell us the bromines are attached to carbon 1 and carbon 2. Trans means one substituent will be dashed back and one will be wedged forward.

How to draw the chair conformation:
1. Draw two slightly offset parallel lines.
2. Draw another pair of parallel lines from the ends of the first pair.
3. Connect with the third set of parallel lines.
4. To draw its ring-flip conformer, just start the first pair of lines at the opposite angle.
See LibreText 9.4 Cyclohexane - A Strain-Free Cycloalkane (How to Draw the Chair Conformation)
Identify the axial and equatorial positions. axial positions in red:
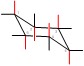

equatorial positions in blue:


Convert your simple line structure to your chair conformation:
1. Match the numbered carbons from your line structure to your template.
2. Recall that substituents wedged forward will be on the higher position and the substituent dashed backwards will be on the lower position.
 to
to 
Identify the more stable conformation. The most stable conformation is the one that has the least number of interactions between substituent atoms. The larger, more electronegative atoms are favoured in the equatorial positions as they will be impacted by less interactions with surrounding atoms.
Complete Solution:
Simple Line Structure:

This molecule is trans meaning one bromine is dashed back and one is wedged forward. These bromines are on a six-carbon ring and are attached to carbons one and two.
Chair conformations:
axial

Both bromines are in axial positions. The bromine on carbon one is in the highest position and the bromine on carbon two is in the lowest position.
equatorial

Both bromines are in equatorial positions. All axial and equatorial positions switch. The bromine on carbon one is in the highest position and the bromine on carbon two is in the lowest position.
is the most stable. It has the least interactions between the bromine groups and surrounding atoms because they are both in equatorial positions.
Answer
The equatorial conformation is the most stable. It has the least interactions between the bromine groups and surrounding atoms because they are both in equatorial positions.
Check your work!
To analyze if your answer is correct, evaluate which conformation has more equatorial positions and given the most space for any larger groups.
Why does this answer make chemical sense?
In the equatorial positions, the larger bromine atoms are the farthest away from all other substituent atoms. The more interactions there are, the more energy it would require and therefore the less stable it would be.
(Original question authored by Lindsay Blackstock, shared under a CC BY-NC-SA 4.0 license)


