5.2: Question 5.E.17 PASS - which central atom makes a polar molecule?
- Page ID
- 452257
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Molecule XF3 has a dipole moment. Is X Boron or Phosphorus?
- Answer
-
X is Phosphorus.
See LibreText 5.1 Molecular Structure and Polarity (5.1.4 Molecular Polarity and Dipole Moment)
- Strategy Map
-
Step Hint 1. Add up the valence electrons for each molecule (BF3 and PF3). 2. Draw the Lewis Structure for each molecule. Recall your steps for drawing Lewis Structures (see LibreText section 4.4):
- Arrange and connect your atoms
- Add lone pairs on terminal atoms until you have used them all
- Recall that Boron is an exception to the octet rule
3. Evaluate bond dipole moments and determine the shape of each molecule. Differences in electronegativities between atoms will cause a bond to be polar. Lone pairs on the central atom influence molecular shape. When looking at the shape of a molecule, if the bond dipole vectors cancel out the molecule will be nonpolar. If the bond dipole vectors do not cancel out the molecule will have a net diploe moment and be polar. 4. Identify any differences between the two molecules that could cause one to be polar. Which molecule has a lone pair on the central atom? What are the shapes of the two molecules?
- Solution
-
Boron as X:
BF3, 24 valence electrons, B will be central atom
Lewis structure
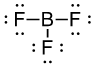
Molecular Geometry, trigonal planar
This molecule is non-polar.
Phosphorus as X:
PF3, 26 valence electrons, P will be central atom
Lewis structure

Molecular Geometry, trigonal pyramid

This molecule is Polar.
Since the molecule XF3 has a dipole moment, X is Phosphorus.
- Guided Solution
-
Download Guided Solution as a pdf
Guided Solution Hint This is a theory type problem that tests you on your ability to identify polarity within molecules by comparing one polar and one non-polar molecule.
See LibreText 5.1 Molecular Structure and Polarity Molecule XF3 has a dipole moment. Is X Boron or Phosphorus?
How does polarity occur within a molecule? Differences in electronegativity between atoms will cause a bond to be polar. When looking at the shape of a molecule, if the bond dipole vectors cancel out the molecule will be nonpolar. If the bond dipole vectors do not cancel out the molecule will have a dipole moment and be polar. How do lone pairs on the central atom impact polarity? Lone pairs on the central atom influence the molecular shape. The resulting positions of terminal atoms must be considered to see if the bond dipole moments are able to cancel Complete Solution:
Boron as X, BF3
Count valence electrons
B has 3 valence electrons, each F has 7, total = 3 + 3(7) = 24 valence electrons
Connect your atoms
B is the central atom, each F is connected to B
Draw the Lewis Structure
Connect the B and each F by a single bond. Then place the remaining electrons as lone pairs around the terminal F atoms. Doing this uses 24 electrons, which matches our count of valence electrons, so the Lewis Structure is complete.
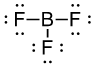
Determine the molecular geometry or shape, and decide if the molecule is polar or nonpolar
Since there are three single bonds of the central atom and no lone pairs, the single bonds arrange themselves equidistant apart in a trigonal planar shape. The bond dipole vectors will all cancel and the molecule will be nonpolar.
 trigonal planar, nonpolar
trigonal planar, nonpolarPhosphorus as X, PF3
Count valence electrons
P has 5 valence electrons, each F has 7, total = 5 + 3(7) = 26 valence electrons
Connect your atoms
P is the central atom, each F is connected to P
Draw the Lewis Structure
Connect the P and each F by a single bond. Then add electrons as lone pairs around the terminal F atoms. Then count how many valence electrons you have used.
At this point we have used 24 valence electrons, and we have two left. Add a lone pair to P, which completes its octet. The Lewis Structure is complete.

Determine the molecular geometry or shape, and decide if the molecule is polar or nonpolar
The lone pair and the three single bonds give a tetrahedral electron pair geometry. Since one is a lone pair the molecular geometry or shape of the molecule which results will be a trigonal pyramid. With this molecular geometry the bond dipole vectors cannot cancel out and the molecule will be polar.
 trigonal pyramid, polar
trigonal pyramid, polarAnswer the question
Since the molecule XF3 has a diploe moment, the molecule must be PF3.
Valence electrons are the outer shell electrons.
The atom that can form the most bonds is the central atom, and is usually the least electronegative atom.
Recall that Boron has a full octet with only six electrons, this is an exception to the octet rule.
Valence shell electron-pair repulsion theory (VSEPR theory) tells us that the electron pairs will arrange themselves to minimize repulsions by maximizing the distance between pairs.
The molecular geometry or shape describes the relative positions of the bonds around a central atom.
Consider the bond dipoles in three dimensions to determine if they cancel.
Check your work!
BF3 is a trigonal planar molecule with no lone pairs and identical terminal atoms so it will be nonpolar. PF3 has one lone pair on the central atom, has a tetrahedral electron pair geometry and a trigonal pyramid shape so it will be polar.
Why does this answer make chemical sense?
For a molecule to be non-polar, the net dipole moment must be zero, meaning the bond dipole moments cancel each other out when the molecular shape is considered.
In the case where atom “X” is Boron there are no lone pairs on the central atom, the three bond dipole moments are equal in magnitude and in opposing directions when molecular shape is considered, the bond vectors cancel and the molecule is nonpolar.
Since Phosphorus has a lone pair on the central atom, even though the bond dipole moments are equal they do not cancel each other out. This means the entire molecule has a dipole-moment and is polar.
If there are no lone pairs on the central atom and the terminal atoms are identical, the molecule will be nonpolar since the bond vectors cancel out.
If there is a lone pair on the central atom and the bond vectors cannot cancel out the molecule will be polar.
(question source from page titled 4.11: Exercises https://chem.libretexts.org/Bookshelves/General_Chemistry/Chemistry_-_Atoms_First_2e_(OpenStax)/04%3A_Chemical_Bonding_and_Molecular_Geometry/4.11%3A_Exercises, shared under a CC BY 4.0 license, authored, remixed, and/or curated by OpenStax, original source https://openstax.org/books/chemistry/pages/7-exercises, Access for free at https://openstax.org/books/chemistry/pages/1-introduction)


