5.3: Question 5.E.59 PASS - identify central atom hybridization
- Page ID
- 452258
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Identify the hybridization of the central atom in each of the following molecules and ions that contain multiple bonds: (parts b-f)
(b) CS2
(c) Cl2CO (C is the central atom)
(d) Cl2SO (S is the central atom)
(e) SO2F2 (S is the central atom)
(f) XeO2F2 (Xe is the central atom)
- Answer
-
(b) sp
(c) sp2
(d) sp3
(e) sp3
(f) sp3d
- Strategy Map
-
Step Hint 1. Create a Lewis Structure of the molecule. See LibreText section 4.4 (Lewis Symblos and Structures) 2. Determine the Electron-Pair geometry of the molecule.
Recall using VSEPR to determine electron pair geometry (see LibreText section 5.1 Molecular Structure and Polarity)
3. Identify the hybridization of the center atom based on the molecule's Electron-Pair geometry. The Hybridization of the center atom will be determined by the electron-pair geometry, not the atom itself. For example, in a linear molecule, the center atoms hybridization will always be sp.
- Solution
-
(b) CS2
16 valence electrons, C central atom
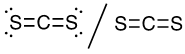
This molecule has a linear electron-pair geometry: sp hybridized
(c) Cl2CO (C is the central atom)
26 valence electrons
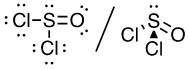
This molecule has a trigonal planar electron-pair geometry: sp2 hybridized
(d) Cl2SO (S is the central atom)
26 valence electrons
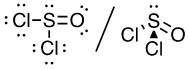
This molecule has a tetrahedral electron-pair geometry: sp3 hybridized
(e) SO2F2 (S is the central atom)
32 valence electrons
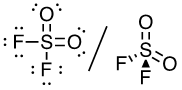
This molecule has a tetrahedral electron-pair geometry: sp3 hybridized
(f) XeO2F2 (Xe is the central atom)
34 valence electrons
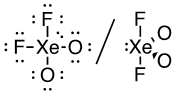
This molecule has a trigonal bipyramidal electron-pair geometry: sp3d hybridized
-
- Guided Solution
-
Download Guided Solution as a pdf
Guided Solution Hint This is a theory problem that requires you to use your knowledge of molecular bonding and identify atomic orbital hybridization. See LibreText 5.3 Hybrid Atomic Orbitals Identify the hybridization of the central atom in each of the following molecules and ions that contain multiple bonds: (only b-f)
(b) CS2
(c) Cl2CO (C is the central atom)
(d) Cl2SO (S is the central atom)
(e) SO2F2 (S is the central atom)
(f) XeO2F2 (Xe is the central atom)
What is the pattern associated with atomic orbital hybridization? The Hybridization of the center atom will be determined by the electron-pair geometry, not the atom itself. For example, in a linear molecule, the center atoms hybridization will always be sp.
Number of hybrid orbitals = Number of orbitals mixed.
Hybrids named by type and number of orbitals mixed.
Complete Solution:
(b) CS2
16 valence electrons, C central atom
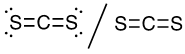
This molecule has a linear electron-pair geometry: sp hybridized
(c) Cl2CO (C is the central atom)
26 valence electrons

This molecule has a trigonal planar electron-pair geometry: sp2 hybridized
(d) Cl2SO (S is the central atom)
26 valence electrons
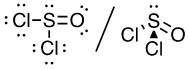
This molecule has a tetrahedral electron-pair geometry: sp3 hybridized
(e) SO2F2 (S is the central atom)
32 valence electrons
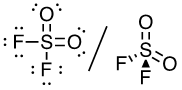
This molecule has a tetrahedral electron-pair geometry: sp3 hybridized
(f) XeO2F2 (Xe is the central atom)
34 valence electrons
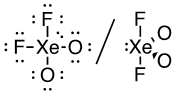
This molecule has a trigonal bipyramidal electron-pair geometry: sp3d hybridized
Recognize this is a linear molecule, two electron domains: s + p = sp Hybridized
The double bond counts as one electron domain.
three electron domains: s + p + p = sp2 hybridized
The double bond counts as one electron domain, the lone pair is one electron domain, and the two single bonds are each one electron domain.
four electron domains: s + p + p + p = sp3 hybridized
four electron domains: s + p + p = sp3 hybridized
five electron domains: s + P + p + d = sp3d hybridized
Check your work!
Verify your Lewis Structure account for all the valence electrons and that you have counted electron domains off the central atom correctly. The name of our electron pair geometry and number of electron domains leads us to the correct hybridization.
Why does this answer make chemical sense?
Hybrid orbitals form due to the covalent bonding within molecules. When two or more atoms create a covalent bond, their atomic orbitals will overlap and form such hybrid orbitals. In this case, we are looking at the hybridization of the center atom to see what kind of hybridization is happening to overlap with all the atoms. When molecules get larger and more atoms are involved, more bonds will be made, and thus more orbitals will be involved in bonding. This is why a linear molecule only uses two orbitals (s + p) and an octahedral molecule uses six orbitals (s + p + p + p + d + d).
Valence electrons are outer shell electrons. Look at the condensed electron configuration.
(question source from page titled 8E: Advanced Theories of Covalent Bonding (Execricse) ttps://chem.libretexts.org/Bookshelves/General_Chemistry/Chemistry_1e_(OpenSTAX)/08%3A_Advanced_Theories_of_Covalent_Bonding/8.E%3A_Advanced_Theories_of_Covalent_Bonding_(Exercises) - Chemistry LibreTexts, shared under a CC BY 4.0 license, authored, remixed, and/or curated by OpenStax, https://openstax.org/books/chemistry/pages/8-exercises, Access for free at https://openstax.org/books/chemistry/pages/1-introduction)


