5.1: Question 5.E.09 PASS - determine electron pair geometry and molecular geometry
- Page ID
- 452261
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)What are the electron-pair geometry and the molecular geometry of each of the following molecules or ions?
a) ClF5
b) ClO2-
c) PCl3
d) SeF4
e) PH2-
- Answer
-
a) Electron-pair geometry: octahedral, Molecular geometry: square pyramidal
b) Electron-pair geometry: tetrahedral, Molecular geometry: bent
c) Electron-pair geometry: tetrahedral. Molecular geometry: trigonal bipyramidal
d) Electron-pair geometry: trigonal bipyramidal, Molecular geometry: seesaw
e) Electron-pair geometry: tetrahedral, Molecular geometry: bent
See LibreText 5.1 Molecular Structure and Polarity (5.1.4 Molecular Polarity and Dipole Moment)
- Strategy Map
-
Step Hint 1. Create a Lewis Structure for the molecule. Recall your steps for drawing Lewis Structures (see LibreText section 4.4):
Arrange and connect your atoms. Add lone pairs on terminal atoms until you have used them all. Recall that Boron is an exception to the octet rule.2. Identify how many total electron domains are attached to the center atom. Electron domains are other atoms and lone pairs attached to the center atom. For example, PCl3 has four electron domains.

3. Identify how many of the electron domains are lone pairs. This will determine the molecules molecular geometry. Lone pairs will repel the other atoms and distort the molecules shape. Example in PCl3 the lone pairs attached to phosphorus cause the Chlorine atoms to be pushed downwards.

- Solution
-
a) ClF5
42 valence electrons, Cl will be central atom.
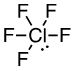
Six electron domains (The center Chlorine atom is attached to five Fluorine’s and has one lone pair).
Electron-pair geometry: octahedral, Molecular geometry: square pyramidal
b) ClO2-
17 valence electrons, Cl will be central atom.
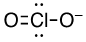
Four electron domains (The center Chlorine atom is attached to two oxygen’s and has two lone pairs).
Electron-pair geometry: tetrahedral, Molecular geometry: bent
c) PCl3
42 valence electrons, P will be central atom

Four electron domains (The center Phosphorus atom is attached to three Chorine’s and has one lone pair).
Electron-pair geometry: tetrahedral. Molecular geometry: trigonal bipyramidal
d) SeF4
44 valence electrons, Se will be central atom.

Five electron domains (The center Selenium is attached to four Fluorine’s and has one lone pair).
Electron-pair geometry: trigonal bipyramidal, Molecular geometry: seesaw
e) PH2-
8 valence electrons, P will be central atom.
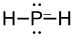
Four electron domains (The center phosphorus atom is attached to two hydrogens and has two lone pairs).
Electron-pair geometry: tetrahedral, Molecular geometry: bent
- Guided Solution
-
Download Guided Solution as a pdf
Guided Solution Hint This is a theory problem where you are asked to determine both the electron-pair geometries (The name for the shape given the number of domains) and the molecular geometries (The name of the shape depending on the electron cloud distortions) for each of the given molecules. See LibreText 5.1 Molecular Structure and Polarity What are the electron-pair geometry and the molecular geometry of each of the following molecules or ions?
To Figure out how many electron domains are in the molecule we must first draw the molecule with all lone pairs and bonds.
We can do this easily by first creating the molecules Lewis structure.
For example, this shows that the molecule has four electron domains:

Why is the electron-pair geometry different from the molecular geometry? Recall that the electron-pair geometry is the basic shape with a certain number of electron domains.
The molecular geometry is the shape the molecule displays of the bonding domains, which is due to electron pair repulsions. If there are lone pairs that attached to the central atom they influence the shape of the molecule.
The molecular geometry can be the same as the electron-pair geometry if the central atom does not have any lone pairs.
For example:

Complete Solution:
a) ClF5
Count valence electrons
42 valence electrons
Connect your atoms
Cl will be central atom.
Draw the Lewis Structure

Count electron domains and bonding domains off the central atom
Six electron domains (The center Chlorine atom is attached to five Fluorine’s and has one lone pair).
Electron-pair geometry: octahedral
Determine the molecular geometry by considering the resulting shape of the bonding domains only, and name the resulting molecular geometry.

Molecular geometry: square pyramidal
b) ClO2-
17 valence electrons, Cl will be central atom.
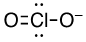
Four electron domains (The center Chlorine atom is attached to two oxygen’s and has two lone pairs).
Electron-pair geometry: tetrahedral

Molecular geometry: bent
c) PCl3
42 valence electrons, P will be central atom.

Four electron domains (The center Phosphorus atom is attached to three Chorine’s and has one lone pair).
Electron-pair geometry: tetrahedral
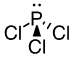
Molecular geometry: trigonal bipyramidal
d) SeF4
44 valence electrons, Se will be central atom.

Five electron domains (The center Selenium is attached to four Fluorine’s and has one lone pair).
Electron-pair geometry: trigonal bipyramidal

Molecular geometry: seesaw
e) PH2-
8 valence electrons, P will be central atom.
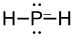
Four electron domains (The center phosphorus atom is attached to two hydrogens and has two lone pairs).
Electron-pair geometry: tetrahedral

Molecular geometry: bent
Valence electrons are the outer shell electrons.
Cl is the least electronegative atom, so it is central.
Total electron domains will determine electron pair geometry.
Valence shell electron-pair repulsion theory (VSEPR theory) tells us that the electron pairs will arrange themselves to minimize repulsions by maximizing the distance between pairs.
The molecular geometry or shape describes the relative positions of the bonds around a central atom.
A wedge indicates a bond coming out of the plane of the screen, a dashed line indicates a bond going in to the plane of the screen.
Add in one electron for the negative charge when counting valence electrons. O has a higher electronegativity so Cl will be the central atom.
Add in one electron for the negative charge when counting valence electrons.
Check your work!
Each of the above have lone pairs on the central atoms so we expect that the molecular geometry will be different than the electron pair geometry in each case and that is what our answers tell us.
Why does this answer make chemical sense?
Previously we had been looking at molecules two dimensionally as if they were flat like a paper. With molecular geometries we are beginning to see that molecules and ions have a three-dimensional shape that move freely. They will move the positions that require the least amount of energy. This means that the outside atoms will move the farthest they can from other negative charges (other atoms and electrons). Lone paired electrons are very repulsive and thus will cause even more distortion within an atom giving it a three-dimensional shape.
In order to determine electron pair geometry you must first start with a correct Lewis Structure. The bonding and lone pairs on the central atom are the electron domains.
Electron pair geometry considers the three dimensional shape of all electron pairs. Molecular geometry considers the three dimensional shape of the bonding pairs.
(question source modified from page titled 4.11: Exercises https://chem.libretexts.org/Bookshelves/General_Chemistry/Chemistry_-_Atoms_First_2e_(OpenStax)/04%3A_Chemical_Bonding_and_Molecular_Geometry/4.11%3A_Exercises, shared under a CC BY 4.0 license, authored, remixed, and/or curated by OpenStax, original source https://openstax.org/books/chemistry/pages/7-exercises, Access for free at https://openstax.org/books/chemistry/pages/1-introduction)


