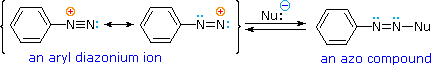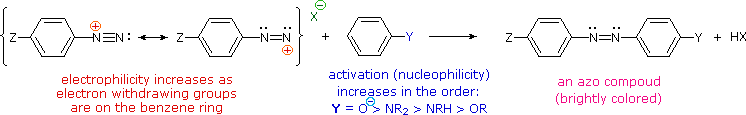24.9: Reactions of Arylamines
- Page ID
- 429745
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)After completing this section, you should be able to
-
- identify the product formed when a given arylamine is reacted with aqueous bromine.
- give an appropriate example to illustrate the high reactivity of arylamines in electrophilic aromatic substitution reactions.
- explain why arylamines cannot be used in Friedel‑Crafts reactions.
-
- show how the problems associated with carrying out electrophilic aromatic substitution reactions on arylamines can be circumvented by first converting the amine to an amide, and illustrate this process with an appropriate example.
- outline a possible synthetic route for the preparation of a given sulfa drug.
- identify the starting material, the necessary organic reagents, inorganic reagents, or both, and the intermediate compounds formed during the synthesis of a given sulfa drug.
- design a multi‑step synthesis for a given compound in which it is necessary to protect the amino group by acetylation.
-
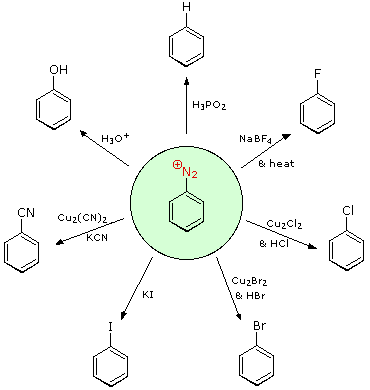
- write a general equation to describe the formation of an arenediazonium salt.
- identify the product formed when a given arenediazonium salt is reacted with any of the following compounds: copper(I) chloride, copper(I) bromide, sodium iodide, copper(I) cyanide, hot aqueous acid, hypophosphorous acid.
- identify the arenediazonium salt, the inorganic reagents, or both, needed to produce a given compound by a diazonium replacement reaction.
-
- illustrate, with appropriate examples, the importance to the synthetic chemist of the overall reaction sequence A nitration, B reduction, C diazotization, and D replacement.
- show how the removal of an amino (or nitro) group from an aromatic ring through the reaction of an arenediazonium salt with hypophosphorous acid (H3PO2) can sometimes be of use in organic synthesis.
-
- write a general equation to represent a diazonium coupling reaction.
- write the detailed mechanism for the coupling reaction which takes place between arenediazonium salts and the electon‑rich aromatic rings of phenols and arylamines.
- identify the product formed from the reaction of a given arenediazonium salt with a given arylamine or phenol.
- identify the arenediazonium salt and the arylamine or phenol needed to prepare a given azo compound.
Make certain that you can define, and use in context, the key terms below.
- arenediazonium salt
- azo compound
- Sandmeyer reaction
- sulfa drug
This section contains a considerable amount of new information. To absorb all of it, you should use the three subsections indicated in the reading: electrophilic aromatic substitution and overreaction of aniline (Objectives 1 and 2), the preparation of diazonium salts and the Sandmeyer reaction (Objectives 3 and 4), and diazonium coupling reactions (Objective 5).
The general process in which an aromatic amine is reacted with acetic anhydride, substituted, and then hydrolyzed is known as “protecting the amino group.”
Sulfa drugs have the general formula
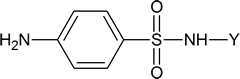
Typical examples are sulfathiazole
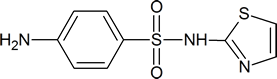
and sulfapyridine
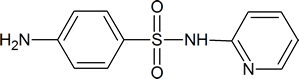
The reagent used to bring about the chlorosulfonation of acetanilide is chlorosulfuric acid
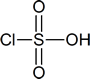
Sulfanilamide itself is toxic to humans, but derivatives of this compound, such as sulfathiazole, are less harmful to humans and are effective in killing many types of bacteria. The drugs work by “deceiving” the bacteria in the following way. To survive, many micro‑organisms use p‑aminobenzoic acid to synthesize folic acid, a coenzyme in a number of biochemical processes. These micro‑organisms cannot distinguish between sulfa drugs and p‑aminobenzoic acid; so, when the drug is administered, the bacteria use it to produce a compound which has a structure similar to that of folic acid, but which is unable to act as a coenzyme in essential biochemical processes. The result is that many of the bacteria’s amino acids and nucleotides cannot be made, and the bacteria die. Amino acids are discussed in Chapter 26; nucleotides are discussed in Section 28.1.
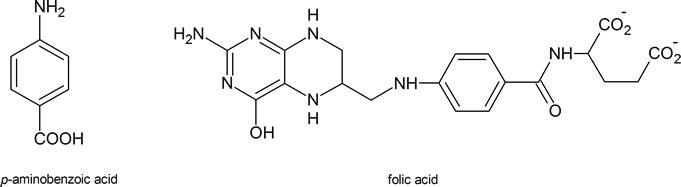
An “arenediazonium salt” is formed by the reaction of an aromatic amine with nitrous acid at 0–5°C, and has the structure shown below.

Alkanediazonium salts are very unstable; therefore, arenediazonium salts are often simply referred to as diazonium salts.
As is mentioned in the textbook, arenediazonium salts are very useful intermediates from which a wide variety of aromatic compounds can be prepared. You should be thoroughly familiar with the use of diazonium salts to prepare each of the classes of compounds. In addition, you should be aware that fluoroarenes can also be prepared from diazonium salts, as follows:
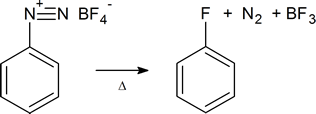
In this case the diazonium salt is prepared using fluoroboric acid, HBF4, and sodium nitrite. The thermal decomposition of the salt, called the Schiemann reaction, can be quite hazardous.
Note that the IUPAC‑preferred name for cuprous chloride is copper(I) chloride; similarly, cuprous cyanide is called copper(I) cyanide.
Hypophosphorous acid has the structure shown below:
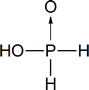
The presence of the letters “azo” in a compound’s name usually implies that a nitrogen‑nitrogen double bond is present in its structure. Azo compounds t in which two aryl groups are joined by an $\ce{-}$N$\ce{=}$N$\ce{-}$ linkage are usually very colourful.
Overreaction of Aniline
Arylamines are very reactive towards electrophilic aromomatic substitution. The strongest activating and ortho/para-directing substituents are the amino (-NH2) and hydroxyl (-OH) groups. Direct nitration of phenol (hydroxybenzene) by dilute nitric acid gives modest yields of nitrated phenols and considerable oxidative decomposition to tarry materials; aniline (aminobenzene) is largely destroyed. Monobromination of both phenol and aniline is difficult to control, with di- and tri-bromo products forming readily.
Because of their high nucleophilic reactivity, aniline and phenol undergo substitution reactions with iodine, a halogen that is normally unreactive with benzene derivatives. The mixed halogen iodine chloride (ICl) provides a more electrophilic iodine moiety, and is effective in iodinating aromatic rings having less powerful activating substituents.
| C6H5–NH2 + I2 + NaHCO3 |  |
p-I–C6H4–NH2 + NaI + CO2 + H2O |
In addition to overreactivity, we have previously seen (Section 16.3) that Friedel-Crafts reactions employing AlCl3 catalyst does not work with aniline. A salt complex forms and prevents electrophilic aromatic substitution.
Both this problem and the aniline overreactivity can be circumvented by first going through the corresponding amide.
Modifying the Influence of Strong Activating Groups
By acetylating the heteroatom substituent on aniline, its activating influence can be substantially attenuated. For example, acetylation of aniline gives acetanilide (first step in the following equation), which undergoes nitration at low temperature, yielding the para-nitro product in high yield. The modifying acetyl group can then be removed by acid-catalyzed hydrolysis (last step), to yield para-nitroaniline. Although the activating influence of the amino group has been reduced by this procedure, the acetyl derivative remains an ortho/para-directing and activating substituent.
C6H5–NH2 + (CH3CO)2O |
pyridine (a base) |
C6H5–NHCOCH3 |
HNO3 , 5 ºC |
p-O2N–C6H4–NHCOCH3 |
H3O(+) & heat |
p-O2N–C6H4–NH |
Sulfa Drug Synthesis
Sulfa drugs are an important group of synthetic antimicrobial agents (pharmaceuticals) that contain the sulfonamide group. The synthesis of sulfanilamide (a sulfa drug) illustrates how the reactivity of aniline can be modified to make possible an electrophilic aromatic substitution. The corresponding acetanilide undergoes chlorosulfonation. The resulting 4-acetamidobenzenesulfanyl chloride is treated with ammonia to replace the chlorine with an amino group and affords 4-acetamidobenzenesulfonamide. The subsequent hydrolysis of the sulfonamide produces the sulfanilamide.
Diazonium Salts: The Sandmeyer Reaction
Aryl diazonium salts are important intermediates. They are prepared in cold (0 º to 10 ºC) aqueous solution, and generally react with nucleophiles with loss of nitrogen. Some of the more commonly used substitution reactions are shown in the following diagram. Since the leaving group (N2) is thermodynamically very stable, these reactions are energetically favored. Those substitution reactions that are catalyzed by cuprous salts are known as Sandmeyer reactions. Fluoride substitution occurs on treatment with BF4(–), a reaction known as the Schiemann reaction. Stable diazonium tetrafluoroborate salts may be isolated, and on heating these lose nitrogen to give an arylfluoride product. The top reaction with hypophosphorus acid, H3PO2, is noteworthy because it achieves the reductive removal of an amino (or nitro) group. Unlike the nucleophilic substitution reactions, this reduction probably proceeds by a radical mechanism.
These aryl diazonium substitution reactions significantly expand the tactics available for the synthesis of polysubstituted benzene derivatives. Consider the following options:
- The usual precursor to an aryl amine is the corresponding nitro compound. A nitro substituent deactivates an aromatic ring and directs electrophilic substitution to meta locations.
- Reduction of a nitro group to an amine may be achieved in several ways. The resulting amine substituent strongly activates an aromatic ring and directs electrophilic substitution to ortho & para locations.
- The activating character of an amine substituent may be attenuated by formation of an amide derivative (reversible), or even changed to deactivating and meta-directing by formation of a quaternary-ammonium salt (irreversible).
- Conversion of an aryl amine to a diazonium ion intermediate allows it to be replaced by a variety of different groups (including hydrogen), which may in turn be used in subsequent reactions.
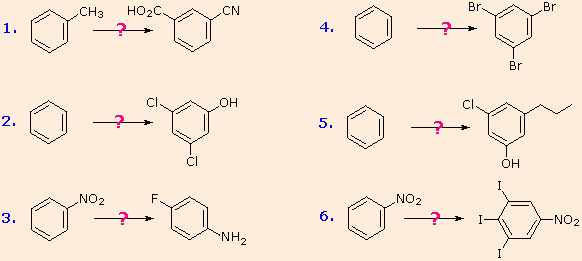
The following examples illustrate some combined applications of these options to specific cases. You should try to conceive a plausible reaction sequence for each. Once you have done so, you may check suggested answers below.
- Answer 1:
-
It should be clear that the methyl substituent will eventually be oxidized to a carboxylic acid function. The timing is important, since a methyl substituent is ortho/para-directing and the carboxyl substituent is meta-directing. The cyano group will be introduced by a diazonium intermediate, so a nitration followed by reduction to an amine must precede this step.
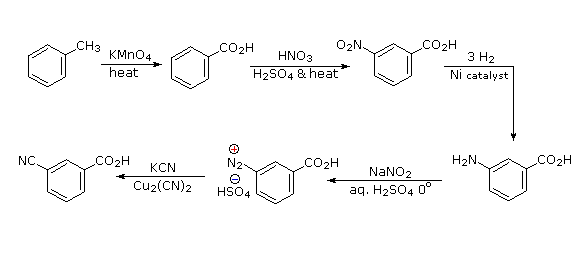
- Answer 2:
-
The hydroxyl group is a strong activating substituent and would direct aromatic ring chlorination to locations ortho & para to itself, leading to the wrong product. As an alternative, the nitro group is not only meta-directing, it can be converted to a hydroxyl group by way of a diazonium intermediate. The resulting strategy is self evident.
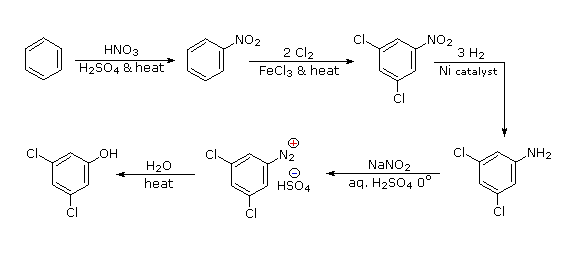
- Answer 3:
-
Selective introduction of a fluorine is best achieved by treating a diazonium intermediate with boron tetrafluoride anion. To get the necessary intermediate we need to make p-nitroaniline. Since the nitro substituent on the starting material would direct a new substituent to a meta-location, we must first reduce it to an ortho/para-directing amino group. Amino groups are powerful activating substituents, so we deactivate it by acetylation before nitration. The acetyl substituent also protects the initial amine function from reaction with nitrous acid later on. It is removed in the last step.
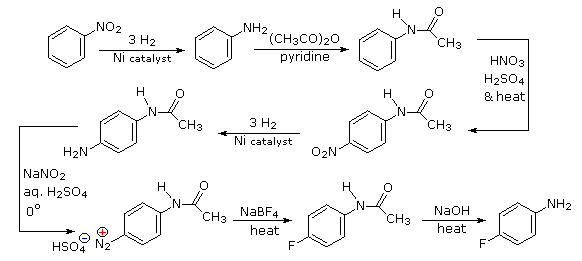
- Answer 4:
-
Polybromination of benzene would lead to ortho/para substitution. In order to achieve the mutual meta-relationship of three bromines, it is necessary to introduce a powerful ortho/para-directing prior to bromination, and then remove it following the tribromination. An amino group is ideal for this purpose. Reductive removal of the diazonium group may be accomplished in several ways (three are shown).
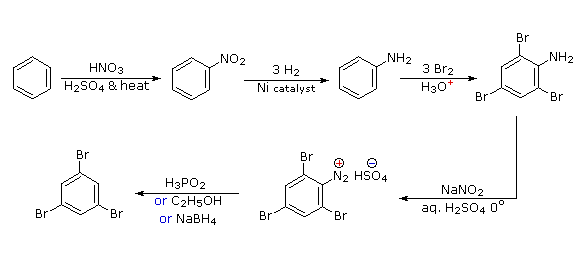
- Answer 5:
-
The propyl substituent is best introduced by Friedel-Crafts acylation followed by reduction, and this cannot be carried out in the presence of a nitro substituent. Since an acyl substituent is a meta-director, it is logical to use this property to locate the nitro and chloro groups before reducing the carbonyl moiety. The same reduction method can be used to reduce both the nitro group (to an amine) and the carbonyl group to propyl. We have already seen the use of diazonium intermediates as precursors to phenols.
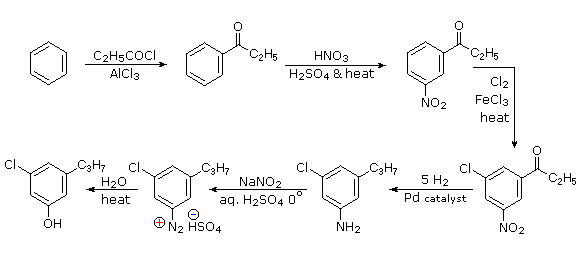
- Answer 6:
-
Aromatic iodination can only be accomplished directly on highly activated benzene compounds, such as aniline, or indirectly by way of a diazonium intermediate. Once again, a deactivated amino group is the precursor of p-nitroaniline (prb.#3). This aniline derivative requires the more electrophilic iodine chloride (ICl) for ortho-iodination because of the presence of a deactivating nitro substituent. Finally, the third iodine is introduced by the diazonium ion procedure.
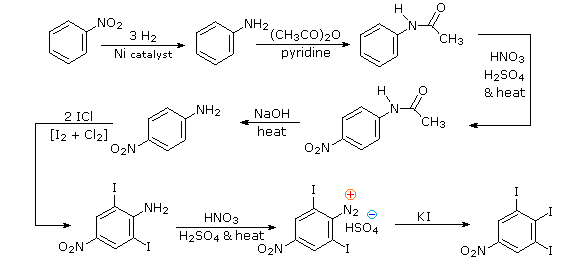
Diazonium Coupling Reactions
A resonance description of diazonium ions shows that the positive charge is delocalized over the two nitrogen atoms. It is not possible for nucleophiles to bond to the inner nitrogen, but bonding (or coupling) of negative nucleophiles to the terminal nitrogen gives neutral azo compounds. As shown in the following equation, this coupling to the terminal nitrogen should be relatively fast and reversible. The azo products may exist as E / Z stereoisomers. In practice it is found that the E-isomer predominates at equilibrium.
Unless these azo products are trapped or stabilized in some manner, reversal to the diazonium ion and slow nucleophilic substitution at carbon (with irreversible nitrogen loss) will be the ultimate course of reaction, as described in the previous section. For example, if phenyldiazonium bisufate is added rapidly to a cold solution of sodium hydroxide a relatively stable solution of sodium phenyldiazoate (the conjugate base of the initially formed diazoic acid) is obtained. Lowering the pH of this solution regenerates phenyldiazoic acid (pKa ca. 7), which disassociates back to the diazonium ion and eventually undergoes substitution, generating phenol.
| C6H5N2(+) HSO4(–) + NaOH (cold solution) |  |
C6H5N2–OH + NaOH (cold) |  |
C6H5N2–O(–) Na(+) |
| phenyldiazonium bisulfate | phenyldiazoic acid | sodium phenyldiazoate |
Aryl diazonium salts may be reduced to the corresponding hydrazines by mild reducing agents such as sodium bisulfite, stannous chloride or zinc dust. The bisulfite reduction may proceed by an initial sulfur-nitrogen coupling, as shown in the following equation.
Ar-N2(+) X(–) |
NaHSO3 |
Ar-N=N-SO3H |
NaHSO3 |
Ar-NH-NH-SO3H |
H2O |
Ar-NH-NH2 + H2SO4 |
The most important application of diazo coupling reactions is electrophilic aromatic substitution of activated benzene derivatives by diazonium electrophiles. The products of such reactions are highly colored aromatic azo compounds that find use as synthetic dyestuffs, commonly referred to as azo dyes. Azobenzene (Y=Z=H) is light orange; however, the color of other azo compounds may range from red to deep blue depending on the nature of the aromatic rings and the substituents they carry. Azo compounds may exist as cis/trans isomer pairs, but most of the well-characterized and stable compounds are trans.
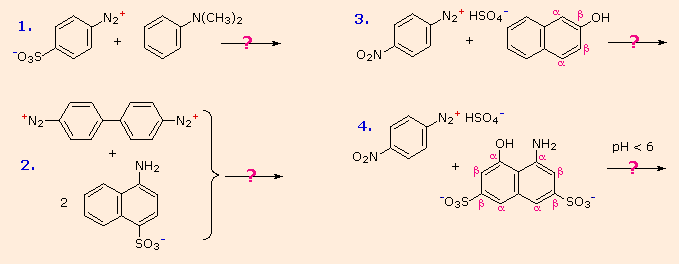
Some examples of azo coupling reactions are shown below. A few simple rules are helpful in predicting the course of such reactions:
- At acid pH (< 6) an amino group is a stronger activating substituent than a hydroxyl group (i.e. a phenol). At alkaline pH (> 7.5) phenolic functions are stronger activators, due to increased phenoxide base concentration.
- Coupling to an activated benzene ring occurs preferentially para to the activating group if that location is free. Otherwise ortho-coupling will occur.
- Naphthalene normally undergoes electrophilic substitution at an alpha-location more rapidly than at beta-sites; however, ortho-coupling is preferred. See the diagram for examples of α / β notation in naphthalenes.
You should try to conceive a plausible product structure for each of the following couplings.