1.9: Alkylation of Acetylide Anions
- Page ID
- 443758
After completing this section, you should be able to
- write an equation to describe the reaction of an acetylide ion with an alkyl halide or an epoxide.
- discuss the importance of the reaction between acetylide ions and alkyl halides or epoxides as a method of extending a carbon chain.
- identify the alkyne (and hence the acetylide ion) and the alkyl halide or epoxide needed to synthesize a given alkyne.
- determine whether or not the reaction of an acetylide ion with a given alkyl halide or epoxide will result in substitution or elimination, and draw the structure of the product formed in either case.
Make certain that you can define, and use in context, the key term below.
- alkylation
The alkylation of acetylide ions is important in organic synthesis because it is a reaction in which a new carbon-carbon bond is formed; hence, it can be used when an organic chemist is trying to build a complicated molecule from much simpler starting materials.
The alkyl halide used in this reaction must be primary. Thus, if you were asked for a suitable synthesis of 2,2-dimethyl-3-hexyne, you would choose to attack iodoethane with the anion of 3,3- dimethyl-1-butyne
rather than to attack 2-iodo-2-methylpropane with the anion of 1-butyne.

The reasons will be made clear in Chapter 3.
Nucleophilic Substitution Reactions of Acetylides
The presence of lone pair electrons and a negative charge on a carbon means acetylide anions are strong bases and strong nucleophiles. Therefore, acetylide anions can attack electrophiles such as alkyl halides or epoxides to cause a substitution reaction. These substitution reactions will be discussed in detail in Chapter 2.
Mechanism
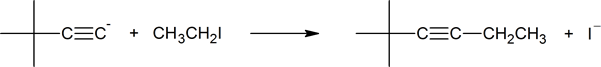
The C-X bonds in 1o alkyl halides are polarized due to the high electronegativity of the halogen. The electrons of the C-X sigma bond are shifted towards the halogen giving it a partial negative charge. This also causes electrons to be shifted away from the carbon giving it a partial positive and making it electrophilic. During this reaction, the lone pair electrons on the acetylide anion attack the electrophilic carbon in the 1o alkyl halide forming a new C-C bond. The formation of this new bond causes the expulsion of the halogen as what is called a leaving group. Overall, this reaction forms a C-C bond and converts a terminal alkyne into a internal alkyne. Because a new alkyl group is added to the alkyne during this reaction, it is commonly called an alkylation.
This substitution reaction is often coupled with the acetylide formation, discussed in the previous section, and shown as a single reaction.
Similar reactions occur with epoxides. Since substitution reactions of acetylide anions are controlled by sterics, substitution occurs at the less substituted side of the epoxide. Ethylene oxide is a very useful two-carbon electrophile that is often used in synthesis. For a broader discussion of epoxide reactions, see Chapter 3.7.
Terminal alkynes can be generated through the reaction of acetylene and a 1o alkyl halide.
Because the acetylide anion is a very strong base, this substitution reaction is most efficient with methyl or primary halides. Secondary, tertiary, or even bulky primary halogens will give alkenes by the E2 elimination mechanism discussed in Section 2.8. An example of this effect is seen in the reaction of bromocyclopentane with a propyne anion. The reaction produces the elimination product cyclopentene rather than the substitution product 1-propynylcyclopentane.
Nucleophilic Addition of Acetylides to Carbonyls
Acetylide anions also add to the electrophilic carbon in aldehydes and ketones to form alkoxides, which, upon protonation, give propargyl alcohols. With aldehydes and non-symmetric ketones, in the absence of chiral catalyst, the product will be a racemic mixture of the two enantiomers. This is similar to what you learned in CHM 222 for addition reactions of Grignard and organolithium reagents to aldehydes and ketones.
The pKa of ammonia is 35. Estimate the equilibrium constant for the deprotonation of pent-1-yne by sodium amide, as shown below.
- Answer
-
Assuming the pKa of pent-1-yne is about 25, then the difference in pKas is 10. Since pentyne is more acidic, the formation of the acetylide will be favored at equilibrium, so the equilibrium constant for the reaction is about 1010.
Synthesize each of the following molecules using an acetylide alkylation reaction.
- Answer
-
Propose a synthetic route to produce trans 2-pentene from propyne and an alkyl halide.
- Answer
-
Using acetylene as the starting material, show how you would synthesize the following compounds.
- Answer
-
Complete the following synthesis.
- Answer
-

