7.4: Step-by-Step Procedures for Melting Point Determination
- Page ID
- 489073
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)There are a variety of methods by which a sample's melting point can be measured, with the newest being electrical probes (e.g. Vernier MeltStation).
Sample Preparation

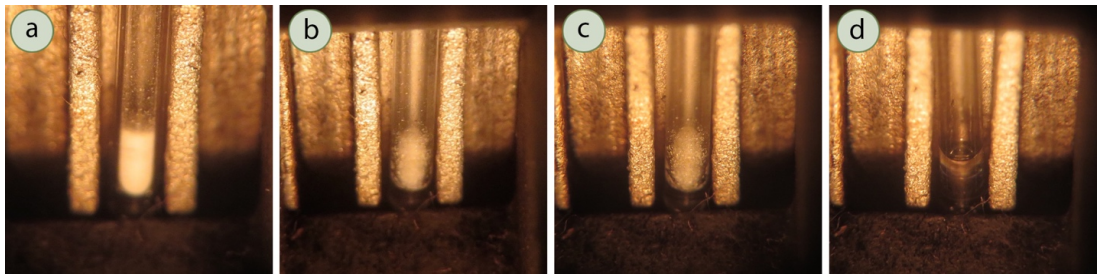
- Obtain a glass capillary melting point tube, which has one end sealed and the other end open. Jab the open end of the tube into a pile of the solid to be analyzed (Figure 6.10a). The solid must be dry or the results will be affected as solvent can act as an impurity and affect the melting range. If the solid is granular, pulverize the solid somewhat before packing.
- Invert the capillary tube and gently tap the tube on the benchtop to cause the solid to fall to the closed end (Figure 6.10b). Then, drop the capillary tube closed side down several times through a long narrow glass tube (Figure 6.10 c). The capillary tube will bounce as it hits the benchtop, and pack the solid into the bottom of the tube. Failure to pack the solid well may cause it to shrink when heating, which can cause confusion as to the correct melting temperature.
- If needed, repeat the previous steps to load the sample until it is a height of \(1\)-\(2 \: \text{mm}\) in the tube (when packed, Figure 6.10d). It is important that the sample be no higher than \(2 \: \text{mm}\) or the melting range will be artificially broad.
Melting Point Apparatus
 Figure 6.11: a) Insertion of capillary sample into the melting point apparatus, b) Control for heating rate, start and end temperatures c) Monitoring of the sample through the viewfinder
Figure 6.11: a) Insertion of capillary sample into the melting point apparatus, b) Control for heating rate, start and end temperatures c) Monitoring of the sample through the viewfinder
- Turn on the apparatus and adjust the setting to an appropriate heating rate.
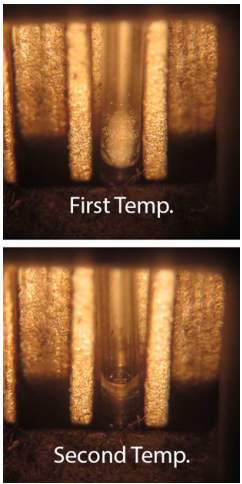
- The initial measure should be taken in a wider range at a faster ramp rate. This is called a "rough melting point". You should start below the expected melting point (literature value) and heated at a ramp rate of 5 °C/minute.
- After the rough melting range is determined, a fine melting point should be determined. It is very important to adjust your starting temperature as you are going to heat at a slower rate to hone in on your melting point range. The starting temperature should be c.a. 5 °C below the starting rough melting point range and you ramp rate should be 1 °C/minute. (i.e., very slowly).
- The end temperature can be set to 10 - 20 °C above the literature melting point since the instrument can be manually stopped once the solid has melted.
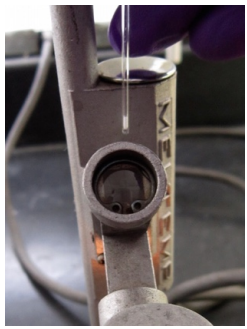
- Insert the capillary tube containing the sample into a slot behind the viewfinder of a melting point apparatus (Figure 6.11a). There are usually three slots in each apparatus, and multiple melting points can be taken simultaneously after gaining experience with the technique.
- Look through the viewfinder (Figure 6.11c) to see a magnified view of the sample in the apparatus, which should be illuminated.
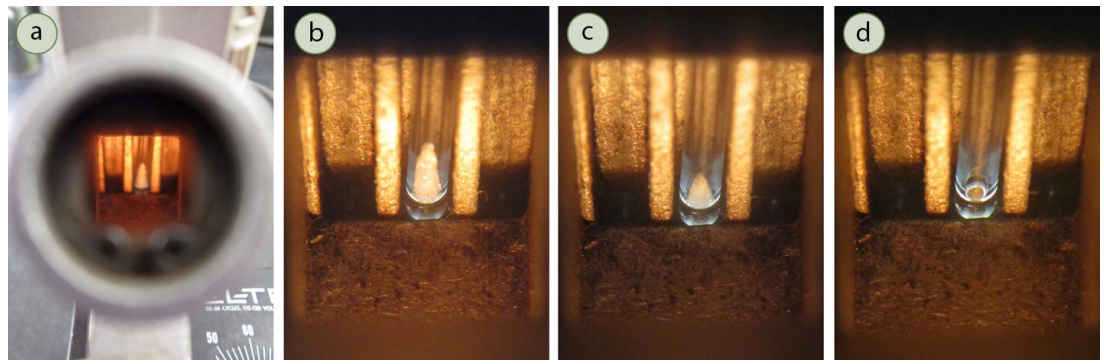
- The solid may be approaching its melting point if the solid is seen pulling away from the walls of the tube to form a cone of solid (Figure 6.12b), which is called "sintering." Melting will normally occur within a few degrees of this point. The solid may also shrink or compact before melting.
Record the first temperature of the melting range with the appearance of the first visible drop of liquid. At first it may seem as if the sides of the solid glisten (Figure 6.13b), and the temperature should be recorded when a droplet is seen on the side or bottom of the tube (a hint of movement will be noticed in the tube, Figure 6.13c).
- Record the second temperature of the melting range when the entire sample has just melted (Figure 6.13d), which occurs when all portions of the opaque solid have turned to a transparent liquid.
- The following unusual situations may occur in the process:
- The sample may begin to darken, which indicates decomposition is occurring before the sample is melting. Take note of the decomposition temperature, as it is sometimes as reliable a reference point as a compound's melting point. Use the letter "d" after a melting point to indicate decomposition (e.g. \(251^\text{o} \text{C}\) d).
- The sample may sublime instead of melting. Sublimation may be noticed by a ring of solid above where the sample is heated. Take note of this behavior in your lab notebook.
- If another melting point trial is to be performed directly after the first, the metal block should be rapidly cooled to at least \(20^\text{o} \text{C}\) below the next melting point by touching it with wet paper towels (Figure 6.11d) or cooling it with a jet of air.
- Repeat the process with a fresh sample after allowing the apparatus to cool. A fresh sample is necessary for a second melting point trial; even if the first sample solidifies after cooling it should not be used again. Differences in crystal structure between the original solid and the previously melted solid could lead to different melting ranges.
Melting Point Summary
 |
 |
 |
 |
|
Load the sample by jabbing the open end of a capillary tube into a pile of the sample. With closed end down, drop the tube down a long hollow tube so that it hits the benchtop and packs the sample into the closed end of the tube. Load the sample to a height of \(2\)-\(3 \: \text{mm}\). |
Place the sample into a slot in the MelTemp. Turn the dial to begin heating. Heat at a medium rate to \(20^\text{o} \text{C}\) below the expected melting point. Then heat very slowly (\(1^\text{o} \text{C}\) every 30 seconds). |
Record the temperature where the first droplet of liquid is seen (there is movement in the tube). Record the second temperature when the entire sample liquefies (the entire sample changes from opaque to transparent). Record a melting range, e.g. \(120\)-\(122^\text{o} \text{C}\). |
If another melting point trial is to be performed, cool the metal block to at least \(20^\text{o} \text{C}\) below the next melting point, by wiping it with a wet paper towel or cooling with a jet of air. |


