9: Oxidation/Reduction Reactions
- Page ID
- 204133
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Objectives
- To determine what is meant by the term "reduction potential".
- To determine the relative reactivity series of metals.
- To determine the cell potential of metal/metal ion combinations.
Introduction
You have already studied acid-base reactions, a very important type of reaction in chemistry. Another very important set of reactions involves another “exchange” process: oxidation–reduction reactions. You will examine several reactions, looking for patterns and relationships. In this experiment you will first make some qualitative observations of the relative reactivity of metals and their common metal cation. Then you will measure the electrical potential between pairs of metals and metal cations to establish the reduction potentials of five unknown metals relative to an arbitrarily chosen metal. This is accomplished by measuring the voltage, or potential difference, between various pairs of half-cells.
A voltaic cell utilizes a spontaneous oxidation-reduction reaction to produce electrical energy. Half-cells are normally produced by placing a piece of metal into a solution containing a cation of the metal (e.g., \(\ce{Cu}\) metal in a solution of \(\ce{CuSO_{4}}\) or \(\ce{Cu^{2+}}\). In this micro-version of a voltaic cell, the half cell will be a small piece of metal placed into a drop of solution on a piece of filter paper. The solution contains the cation of the solid metal. A porous barrier or a salt bridge normally separates the two half-reactions. Here, the salt bridge will be several drops of aqueous NaNO3 placed on the filter paper between the two half cells. Using a voltmeter, the (+) lead makes contact with one metal and the (–) lead with another. If a positive voltage is recorded on the screen, you have connected the cell correctly. The metal attached to the (+) lead is the cathode (reduction) and thus has a higher, more positive, reduction potential. The metal attached to the (–) lead is the anode (oxidation) and has the lower, more negative, reduction potential. If you get a negative voltage reading, then you must reverse the leads.
By comparing the voltage values obtained for several pairs of half-cells, and by recording which metal made contact with the (+) and (–) leads, you can establish the reduction potential sequence for the five metals in this lab.
Pre Lab Video
Procedure
Safety and Waste Disposal
- The solutions must be disposed of in the hazardous waste container. Filter paper must be disposed of in the solid waste container.
Part I: Interactions of Metals and Metal Ions
Step 1
In your lab notebook create a data table with the ions on the left in a column and the metals in a row across the top.
Step 2
 Fill four tubes with 1 mL 0.20 molar copper nitrate solution.
Fill four tubes with 1 mL 0.20 molar copper nitrate solution.
Step 3
 Clean the longer strips of \(\ce{Cu}, \ce{Zn}, \ce{Pb}, \ce{Mg}\; \text{and}\; \ce{Ag}\) metals by gently rubbing the last inch of each strip with sandpaper.
Clean the longer strips of \(\ce{Cu}, \ce{Zn}, \ce{Pb}, \ce{Mg}\; \text{and}\; \ce{Ag}\) metals by gently rubbing the last inch of each strip with sandpaper.
Step 4
 Submerge the cleaned end of the strip in the solutions for one to two minutes and then record your observations in your table.
Submerge the cleaned end of the strip in the solutions for one to two minutes and then record your observations in your table.
Step 5
Dispose of the metal nitrate solution and clean your test tubes. Clean the metal surfaces and reuse for steps 6-9.
Step 6
Repeat steps 2 - 5 with 0.20 molar zinc nitrate solutions.
Step 7
Repeat steps 2 - 5 with 0.20 molar lead nitrate solutions.
Step 8
Repeat steps 2 - 5 with 0.20 molar silver nitrate solutions.
Step 9
Repeat steps 2 - 5 with 0.20 molar magnesium nitrate solutions.
Step 10
Collect the metal pieces that remain, rinse them with distilled water and return them to their appropriate containers. Dump any remaining solutions into the waste containers in the hood.
Step 11
Write the chemical reaction for those experiments where a reaction occurred. If no reaction occurred write “No reaction”.
Step 12
Which metal is the most reactive? Which metal ion is the most reactive?
Step 13
Mental Model: Choose one of the reactions that occurred and draw a picture of what is occurring in the test tube.
Part II Voltaic Cells
Step 1
Obtain a piece of filter paper cut it into strips to use as salt bridges.
Step 2
 Add 1-2 mL of each metal nitrate \(\ce{Cu(NO_{3})_{2}}, \ce{Zn(NO_{3})_{2}}, \ce{Pb(NO_{3})_{2}}, \ce{AgNO_{3}}\; \text{and}\; \ce{Mg(NO_{3})_{2}}\) solutions to a different well in a well plate.
Add 1-2 mL of each metal nitrate \(\ce{Cu(NO_{3})_{2}}, \ce{Zn(NO_{3})_{2}}, \ce{Pb(NO_{3})_{2}}, \ce{AgNO_{3}}\; \text{and}\; \ce{Mg(NO_{3})_{2}}\) solutions to a different well in a well plate.
Step 3
Add several drops of 1 M \(\ce{NaNO}_{3}\) to each filter paper strip. Place one end in the well containing the copper solution and the other end in the well with the zinc solution.
Step 4
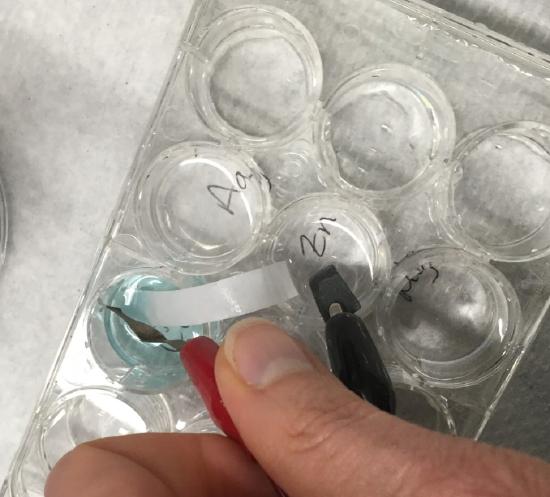 Clip metal copper into one alligator clips and zinc in the other. Place the metals in their respective solutions. If the voltage displayed in the meter is negative, then reverse the leads.
Clip metal copper into one alligator clips and zinc in the other. Place the metals in their respective solutions. If the voltage displayed in the meter is negative, then reverse the leads.
Step 5
With a positive voltage displayed, wait about five seconds to take a voltage reading, and record the value. Be sure that you correctly label your data as you collect it. Also record which metal is the red (+) terminal and which is black (–). Use the same procedure and measure the potential of the other three cells, continuing to use Cu as the reference electrode.
Step 6
Using the Voltage Probe, measure the potential of the remaining half-cell combinations. You should have a total of 10 voltages when you have completed steps 4 and 5. If the \(\ce{NaNO}_{3}\) salt bridge solution has dried, you may have to re-moisten it. Record each measured potential. To complete these measurements, you will connect the metals in the remaining combinations i.e. \(\ce{Zn}\; \text{with}\; \ce{Pb}, \ce{Zn}\; \text{with}\; \ce{Ag}\), etc. You should have 10 measurements when you are done.
Step 7
When you have finished, rinse each piece of metal with tap water. Dry it and return it to the correct container. Remove the filter paper from the well plate and discard it in the solid waste container provided.
Observations
| \(\ce{Cu}\) | \(\ce{Zn}\) | \(\ce{Pb}\) | \(\ce{Mg}\) | \(\ce{Ag}\) | |
| \(\ce{Cu^{2+}}\) | |||||
| \(\ce{Zn^{2+}}\) | |||||
| \(\ce{Pb^{2+}}\) | |||||
| \(\ce{Mg^{2+}}\) | |||||
| \(\ce{Ag^{+}}\) |
| Red | Black | Voltage |
|---|---|---|

