The Oxygen-Evolving Center (OEC) of Photosystem II
- Page ID
- 98125
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Photosynthesis is the process by which plants make energy from the use of chlorophyll and light. It is the process that oxidizes (removes electrons from) water to produce dioxygen and sustains all life on earth.1 It sustains all life through the food chain. Light from the sun initiates photosynthesis in plants, which is turned into energy. Animals eat plants in order to receive energy. Humans can eat those animals or vegetarians can eat the plants and the energy gets transferred again. Eukaryotic cells have an organelle, called a chloroplast, that harvests sunlight and produces energy in the form of ATP. Inside the chloroplast there are stacks called grana and cytoplasm called stroma. Inside the grana (also known as thylakoids) are Photosystem I and II.2 The process through which sunlight gets transferred to Photosystem II is depicted in Figure 1 below. Photosystem II (PSII) is the first major complex in the photosynthetic electron transport chain. It is the only molecule in photosynthesis that can produce dioxygen from water and light.
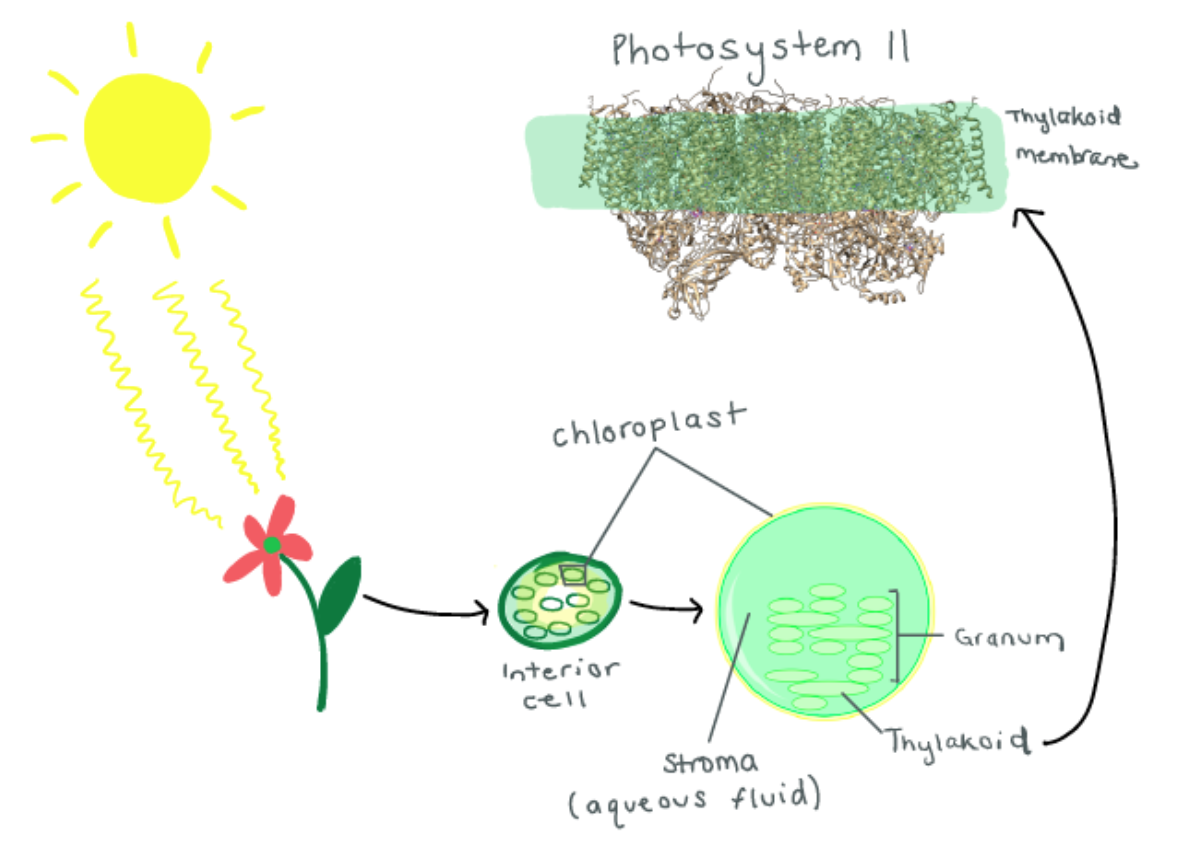
Studying Photosystem II is of particular interest right now for engineers developing photovoltaic solar panels to harvest light energy and use it as a renewable resource. Photosystem II has the most efficient light transformative capability on earth. If engineers and scientists could break down water splitting in the molecule and apply it to cell potential of photovoltaic cells then solar panels could have the possibility of reaching nearer to 100% efficiency.
The sunlight energy obtained by Photosystem II is used to extract electrons from water molecules through certain proteins and enzymes. Two water molecules break into oxygen gas and hydrogen ions, and the freed oxygen gas is the source of oxygen available for us to breath.3
This can be displayed in the chemical Equation 1:
1) 2H2O → O2 + 4e- + 4H+ Reduction Potential=0.815V
The hydrogen ions produced in this reaction are later used to power up ATP synthesis. The four electrons are transferred in the process are passed down a chain of electron-carrying proteins. These electrons are used to pump the hydrogen ions across the membrane, which give even more power to ATP synthesis. Photosystem I helps the electrons along the way to the final destination of photosynthesis. The electrons are finally delivered to enzymes that produce sugar from water and carbon dioxide (also known as the Calvin cycle).1 The photosynthesis chain can be depicted in Figure 2 below.
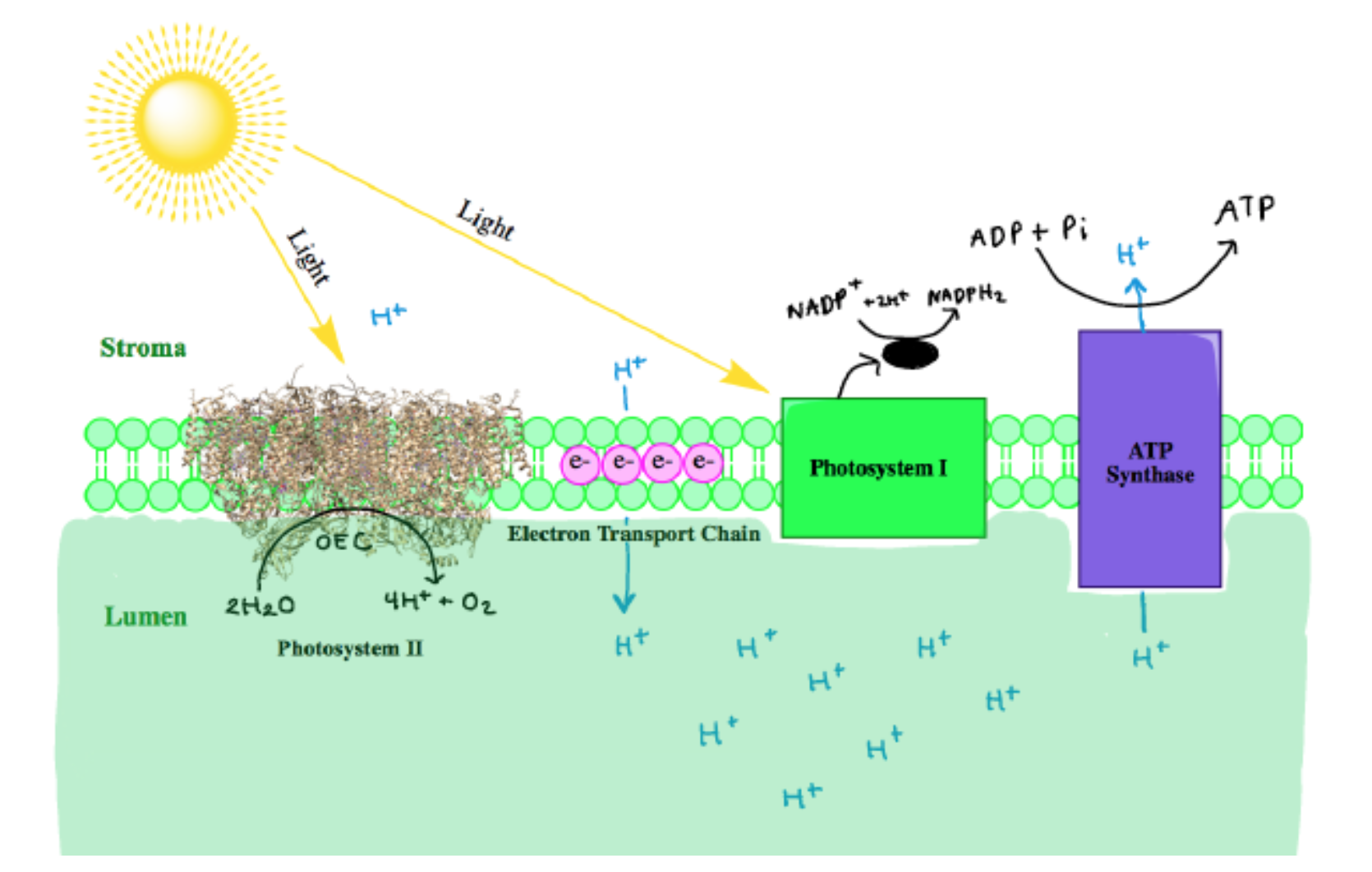
At the reaction center of PSII is the oxygen evolving center (OEC). It is proposed that the metal-oxygen cluster is a cubane shape with the formula Mn3CaO4.4 There are two OECs present in each functional unit of Photosystem II, as shown below in Figure 3. The OEC is responsible for oxidizing 2 H2O to O2 (Equation 1 above).5
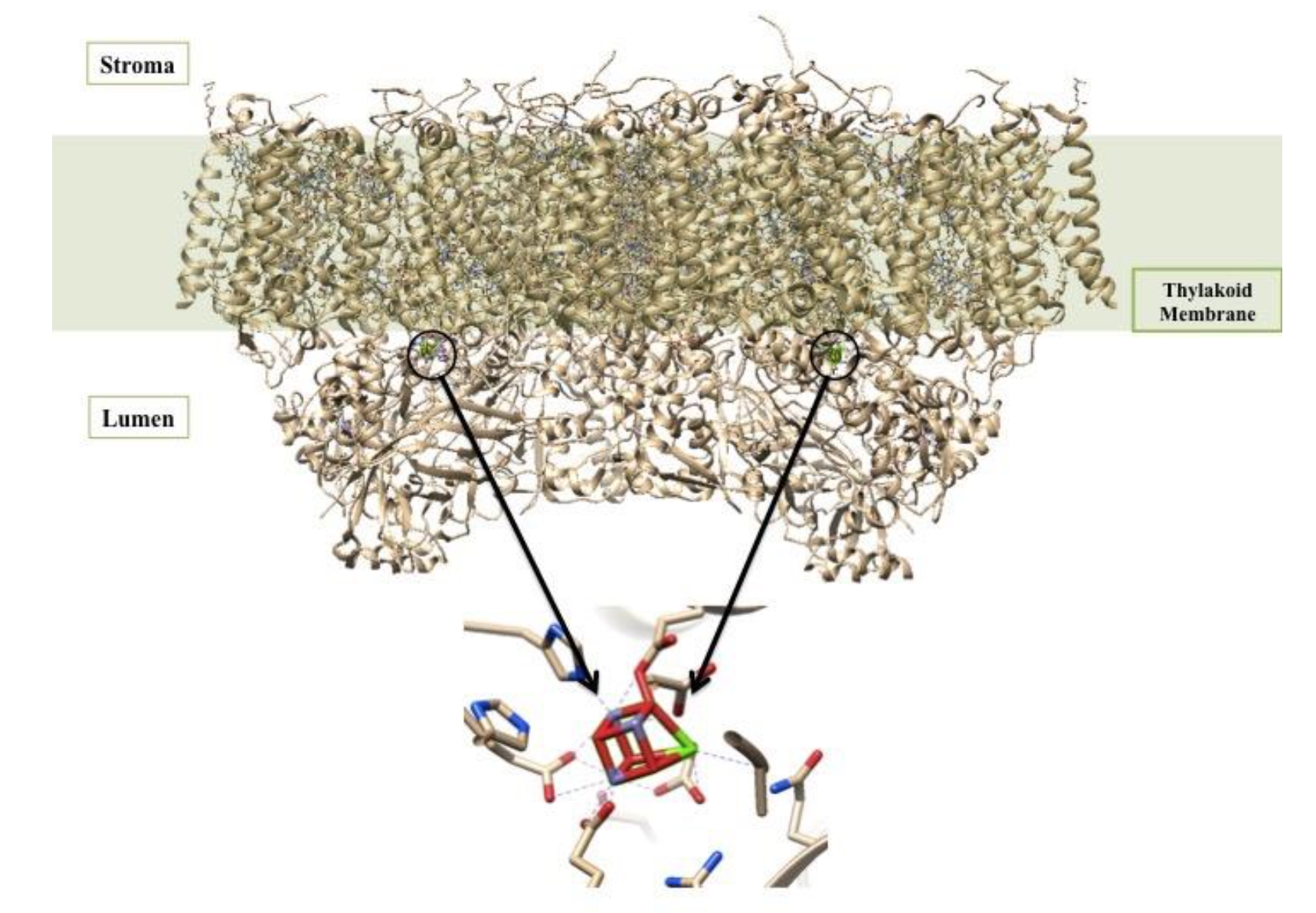
The OEC is held in the protein by histidine, aspartate, glutamate, and alanine side chains.1 In this way, the protein is acting like a multi dentate chealtor. The chelate effect occurs when a ligand has the ability to bind to a metal ion through multiple donor atoms. When the ligand binds through more than one atom it is termed a chelating ligand (or chelator), and a ligand that binds through more than one donor atom it is termed a polydentate system. Chelating ligands typically have lone pairs that can bind to metal ions on two or more of its atoms. There is an energy benefit due to entropy in the chelate effect called the entropic benefit. This comes into play in the OEC and increases the stability of the complex resulting in a greater entropic advantage. The OEC in Photosystem II is a cubane cluster of multiple ions, including manganese and calcium ions, that are bridged through oxygen ions.4 The structure of the OEC is depicted in Figure 4 below. The OEC is bound to the protein through amino acid side chains, all which bind to the OEC directly through the manganese and oxygen ions. Although this is not a simple case of one metal ion being chelated through multiple donor groups, if the OEC is treated as one unit, then the protein can be considered a polydentate system.
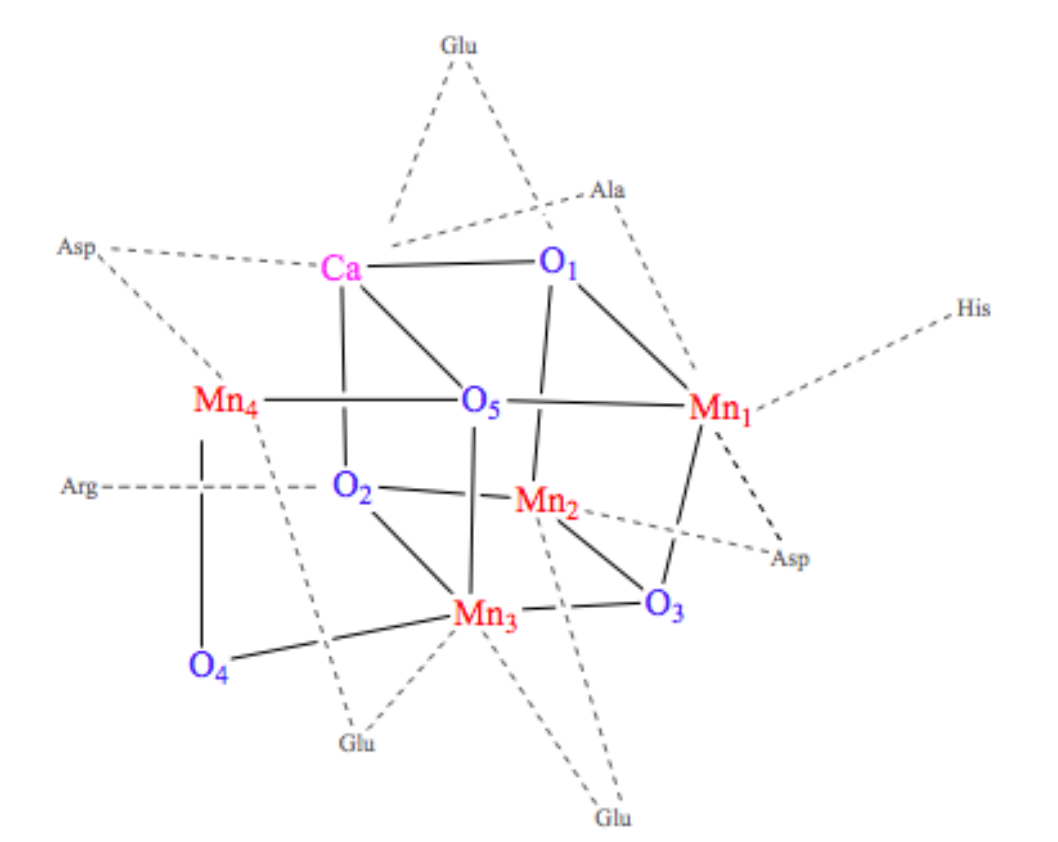
Each manganese in the cubane cluster of the OEC has different ligands. The geometry around each individual metal center is octahedral, meaning they are each coordinated by six ligands. Mn1 has an Asp, three oxygen that are part of the cube, and two possible water molecules as its ligands. Mn2 has Glu, His, three oxygens that are part of the cube, and a possible water molecule as its ligands. Mn3 has Glu as a ligand, which could be one or two coordinated, and three oxygens that are part of the cube as its other ligands. Mn4 has Glu, Asp, a bicarbonate acting as a tridentate bridge with Ca+2, and two water molecules as its ligands. The calcium has the bicarbonate tridentate as a ligand and a carbonyl of an Ala as another ligand. The other ligands of calcium are water or hydroxide ions.6
As stated previously, the metal centers in Photosystem II are manganese and calcium.6 Ca is only found as Ca2+ and its d-electron count is 0. Mn is found in the metal oxidation states of Mn2+, Mn3+, Mn4+, Mn5+.7 Mn2+ has a d electron count of 5, Mn3+ has a d-electron count of 4, Mn4+ has a d electron count of 3, and Mn5+ has a d electron count of 2. All first row transition metals (Mn included) are high spin. High spin correlates to a small delta and contributes to a weak field. Only d4-d7 transition metals can contribute to a high or low spin. Mn4+ and Mn5+ are d3 and d2 metals respectively so they do not have high or low spin states for octahedral geometry. The oxygens in place help stabilize these highly positive Mn ions. Mn2+ and Mn3+ both contribute to a high spin. In an experiment, all Mns were reduced to Mn2+. The Mn2+ came out of the complex because it was labile and had 0 ligand field stabilization energy (LFSE).6
The LFSE splitting diagrams for the five metals in the OEC and the calculations for LFSE can be seen in Figure 5 below. The more negative the value for LFSE the more stable it is. Therefore, Mn4+ is the most stable because it has a LFSE value of -1.2. Mn2+ and Ca2+ both have a LFSE value of 0 so they are the least stable. However, they are the most labile meaning they are very fast to react. Overall, if there are more electrons in the antibonding eg orbitals (seen in Figure 5 below) then the metal is more labile. Mn3+ has a LFSE value of -0.6 and Mn5+ has an LFSE value of -0.8. These two oxidation states are relatively stable and not very labile.
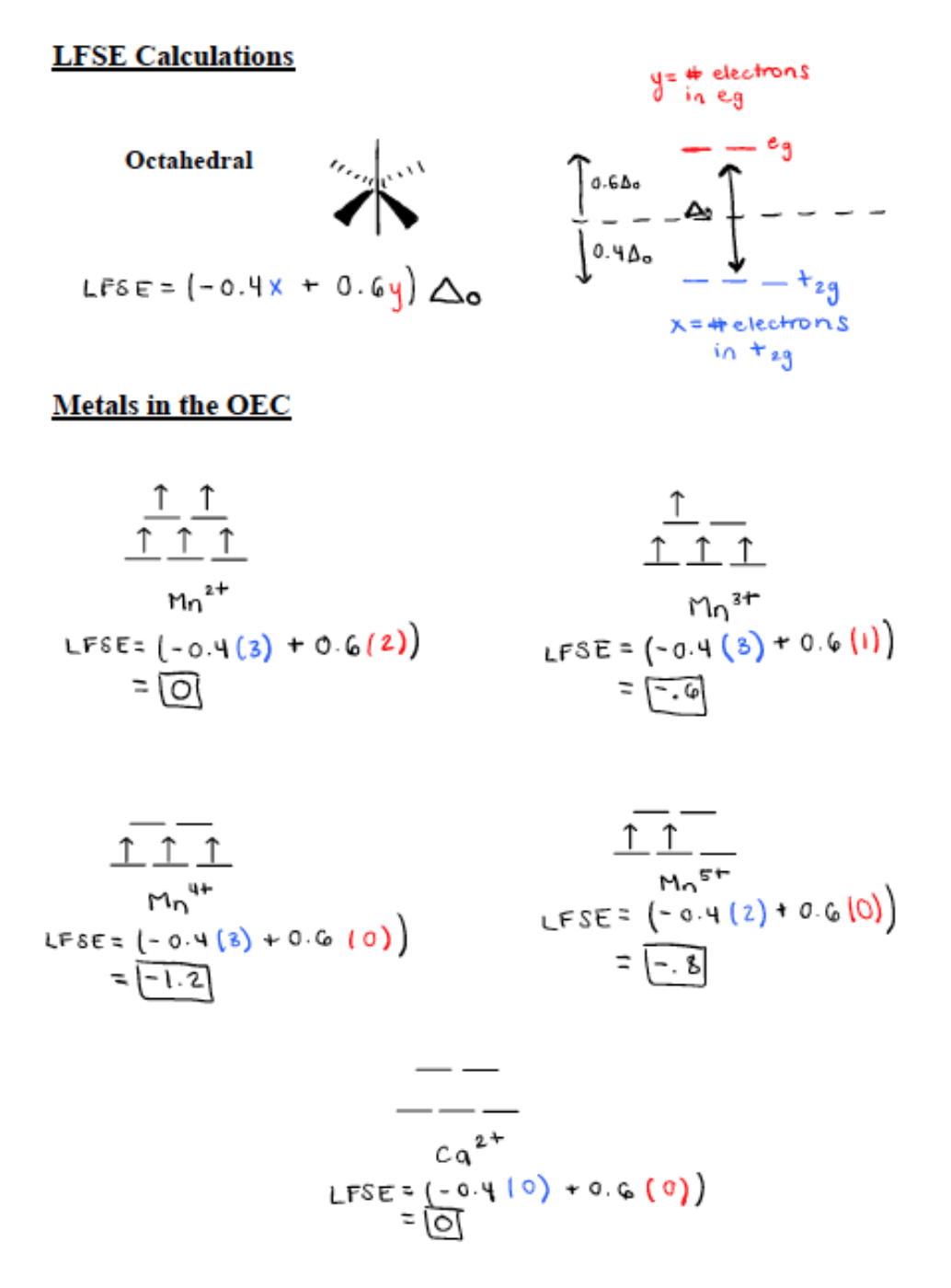
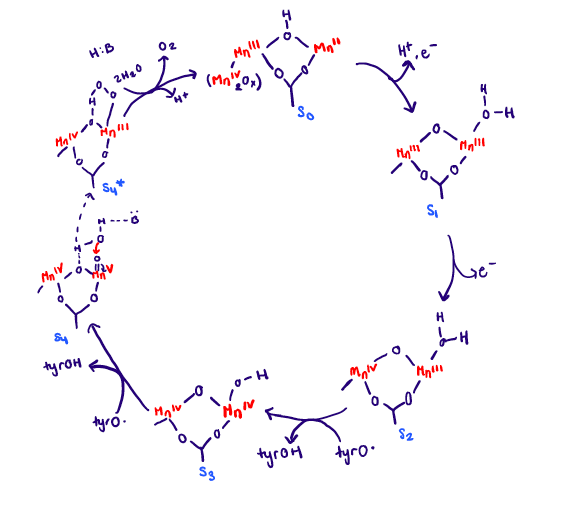
The wide range of oxidation states of Mn is an advantage for its role in water oxidation of PSII. How this exactly happens is through the photocatalytic process. The OEC is oxidized in a series of oxidation steps losing one electron at a time at each step. There are five states that have been observed spectroscopically and they are S0, S1, S2, S3, S4.3 The full cycle can be seen in Figure 6 below. UV-visible spectroscopy have showed changes in oxidation state of Mn2+ to Mn3+ and Mn3+ to Mn4+ throughout the cycle.
The photocatalytic process (water splitting) will be described in simplistic terms. The OEC is oxidized in a series of oxidation steps, and this happens at the same time the chlorophyll complex (P680) gets excited from light. Four photons must be absorbed in order to excite the P680. The MnIV2Ox group is unaltered throughout the cycle so it not shown besides in the S0 state. From the S0 state to the S1 state there is a redox reaction taking place, and Mn2+ is oxidized to Mn3+. The electron goes to P680. From the S1 state to the S2 state there is another redox reaction taking place. Mn3+ is oxidized to Mn4+, and the electron lost also goes to P680. From the S2 state to the S3 state there is another redox reaction taking place, but the electron is transferred through a tyrosine radical. The tyrosine is present to help transfer the electrons from the OEC to P680. It acts almost as a bridge making the two dependent on each other. From the S3 state to the S4 state there is a ligand dissociation, with an unknown base. Finally from the S4 state to the S0 state there is a ligand exchange present where the O2 is released. This whole process is happens at light speed. The final result is that it produces a dioxygen and a hydrogen ion. The roles of Ca2+ are uncertain in the OEC cycle. It is important to note that the OEC acts a redox catalyst throughout the cycle, and also as an electron transport center when the electrons from the OEC get passed to P680.
As mentioned, there lies a special pair of chlorophyll molecules (P680) at the center of PSII along with the OEC. The special pair of chlorophyll molecules are Chlorophyll A and B, and they make up the P680 complex. They are special because they act as excitonic dimer, meaning they behave in function as a single entity.8 The 680 refers to the complex’s absorption maximum in the visible light spectrum (680 nm= red absorption). It reflects green light around 500 nm, which is why we see plants as green.The reduction potential of P680 is very high (around 1.2 to 1.4 V). This is required of the water splitting reaction (S-cycle) in the OEC for oxidizing water into O2 and H+.9 The reduction potential of the dioxygen is 0.815 V (see Equation 1 above). Since this reduction potential is less than the reduction potential of P680, the four electrons are from the OEC are easily transferred to the P680. Figure 7 below gives the reduction potentials of relevant parts of PSII and the reduction potential of Photosystem I.3 The arrows show which way the electrons flow. When they point down the flow is spontaneous and when they point up the flow is non-spontaneous.
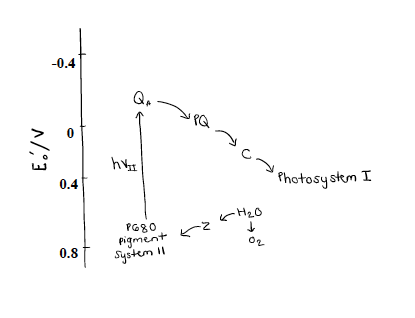
The Z represents the OEC along with the neighboring tyrosine residue. As the OEC enters into the photocatalytic process it passes its electrons freely to P680. As the photon of light is absorbed by P680 it gets excited and non-spontaneously transfers the four electrons to Quinone A (QA). QA is bound to plastoquinone (PQ). QA, PQ, and C are all contribute to the electron transport chain that pass the electrons onto Photosystem I.3 This all happens at speed of light, and explains why photosynthesis can sustain life on earth. It all starts with the complex molecule of Photosystem II, which scientists and engineers are racing to synthetically mimic in order to solve the energy crisis.
Sources
(1) Goodsell, D. S. Photosystem II. RCSB Protein Data Bank 2004.
(2) Mason, K. A.; Losos, J. B.; Singer, S. R.; Raven, P. H. Biology, Eleventh edition.; McGraw-Hill Education: New York, NY, 2017.
(3) Metals and Life; Crabb, E., Moore, E., Open University, Eds.; RSC Pub: Cambridge, U.K, 2010.
(4) Photosystem II. Wikipedia; 2017.
(5) Biological inorganic chemistry structure and reactivity; University Science Books: Sausalito, California, 2007.
(6) Atkins, P. W. Shriver & Atkins’ Inorganic Chemistry; W.H. Freeman and Co.: New York, 2010.
(7) VCAC: Cellular Processes: Photosystem II: First Look http://vcell.ndsu.edu/animations/photosystemII/first.htm (accessed Apr 4, 2018).
(8) Raszewski, G.; Diner, B. A.; Schlodder, E.; Renger, T. Spectroscopic Properties of Reaction Center Pigments in Photosystem II Core Complexes: Revision of the Multimer Model. Biophysical Journal 2008, 95 (1), 105–119.
(9) Ferreira, K. N.; Iverson, T. M.; Maghlaoui, K.; Barber, J.; Iwata, S. Architecture of the


