4.3: Naming stereoisomers
- Page ID
- 225780
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Rules for assigning an R/S designation to a chiral center
1: Assign priorities to the four substituents, with #1 being the highest priority and #4 the lowest. Priorities are based on the atomic number.
2: Trace a circle from #1 to #2 to #3.
3: Determine the orientation of the #4 priority group. If it is oriented into the plane of the page (away from you), go to step 4a. If it is oriented out of the plane of the page (toward you) go to step 4b.
4a: (#4 group pointing away from you): a clockwise circle in part 2 corresponds to the R configuration, while a counterclockwise circle corresponds to the S configuration.
4b: (#4 group pointing toward you): a clockwise circle in part 2 corresponds to the S configuration, while a counterclockwise circle corresponds to the R configuration.
We’ll use the 3-carbon sugar glyceraldehyde as our first example. The first thing that we must do is to assign a priority to each of the four substituents bound to the chiral center. We first look at the atoms that are directly bonded to the chiral center: these are H, O (in the hydroxyl), C (in the aldehyde), and C (in the CH2OH group).
Assigning R/S configuration to glyceraldehyde:
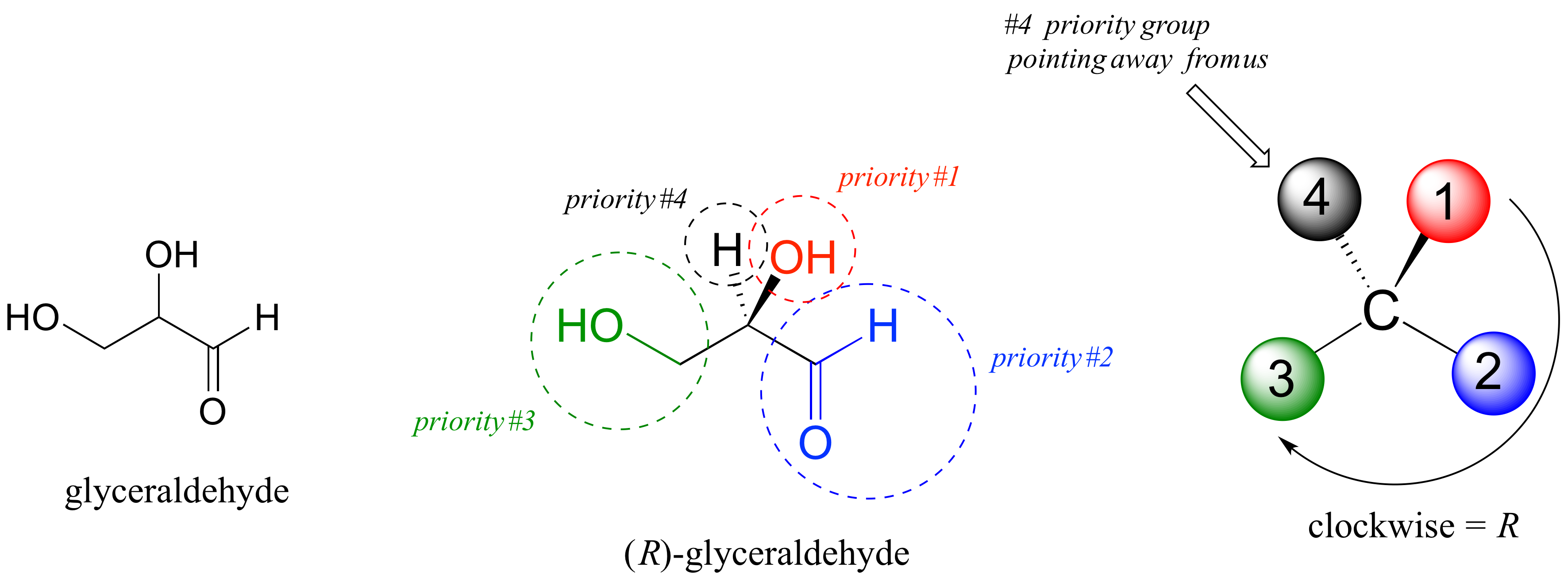
Two priorities are easy: hydrogen, with an atomic number of 1, is the lowest (#4) priority, and the hydroxyl oxygen, with atomic number 8, is priority #1. Carbon has an atomic number of 6. Which of the two ‘C’ groups is priority #2, the aldehyde or the CH2OH? To determine this, we move one more bond away from the chiral center: for the aldehyde we have a double bond to an oxygen, while on the CH2OH group we have a single bond to an oxygen. If the atom is the same, double bonds have a higher priority than single bonds. Therefore, the aldehyde group is assigned #2 priority and the CH2OH group the #3 priority.
With our priorities assigned, we look next at the #4 priority group (the hydrogen) and see that it is pointed back away from us, into the plane of the page – thus step 4a from the procedure above applies. Then, we trace a circle defined by the #1, #2, and #3 priority groups, in increasing order. The circle is clockwise, which by step 4a tells us that this carbon has the ‘R’ configuration, and that this molecule is (R)-glyceraldehyde. Its enantiomer, by definition, must be (S)-glyceraldehyde.
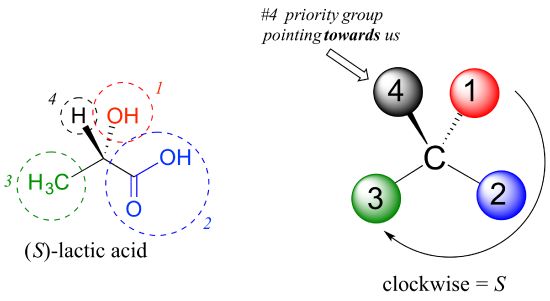
Next, let’s look at one of the enantiomers of lactic acid and determine the configuration of the chiral center. Clearly, H is the #4 substituent and OH is #1. Owing to its three bonds to oxygen, the carbon on the acid group takes priority #2, and the methyl group takes #3. The #4 group, hydrogen, happens to be drawn pointing toward us (out of the plane of the page) in this figure, so we use step 4b: The circle traced from #1 to #2 to #3 is clockwise, which means that the chiral center has the S configuration.
The drug thalidomide is an interesting – but tragic – case study in the importance of stereochemistry in drug design. First manufactured by a German drug company and prescribed widely in Europe and Australia in the late 1950’s as a sedative and remedy for morning sickness in pregnant women, thalidomide was soon implicated as the cause of devastating birth defects in babies born to women who had taken it. Thalidomide contains a chiral center, and thus exists in two enantiomeric forms. It was marketed as a racemic mixture: in other words, a 50:50 mixture of both enantiomers.
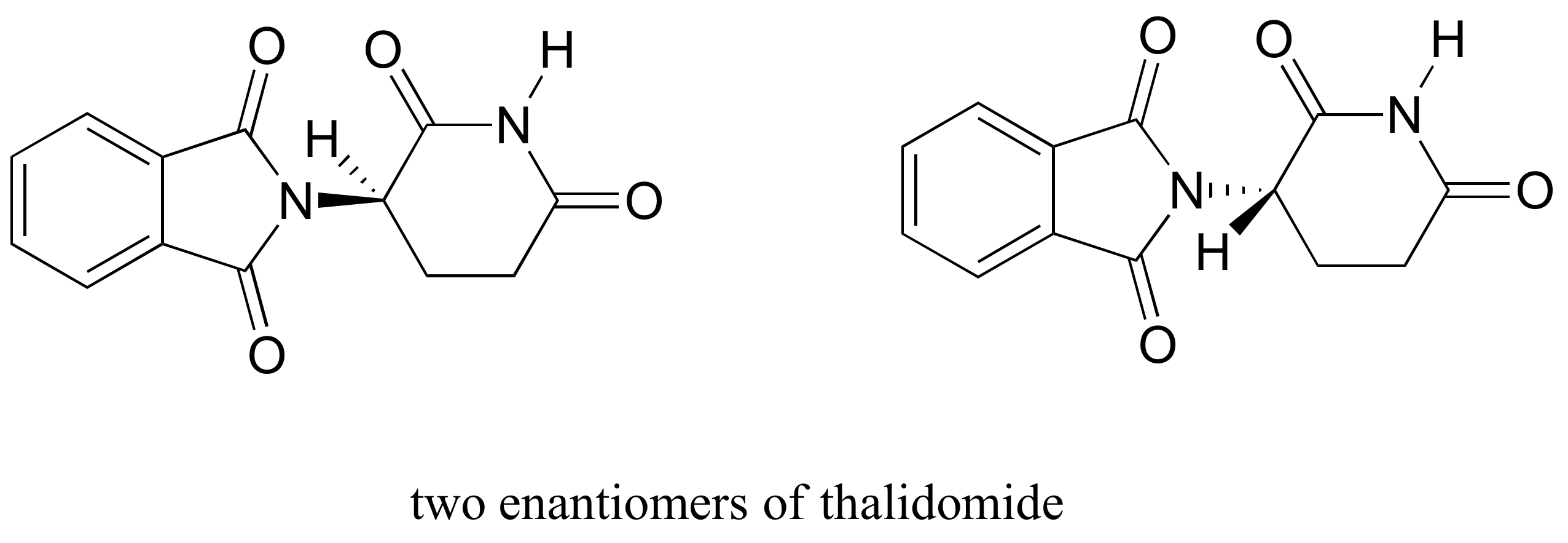
Let’s try to determine the stereochemical configuration of the enantiomer on the left. Of the four bonds to the chiral center, the #4 priority is hydrogen. The nitrogen group is #1, the carbonyl side of the ring is #2, and the –CH2 side of the ring is #3.
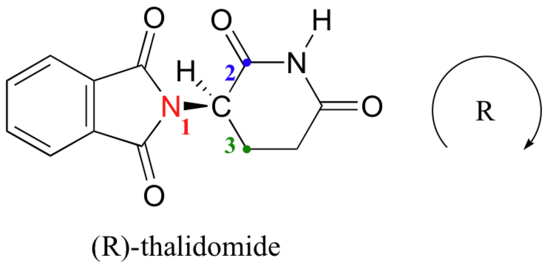
The hydrogen is shown pointing away from us, and the prioritized substituents trace a clockwise circle: this is the R enantiomer of thalidomide. The other enantiomer, of course, must have the S configuration.
Although scientists are still unsure today how thalidomide works, experimental evidence suggests that it was actually the R enantiomer that had the desired medical effects, while the S enantiomer caused the birth defects. Even with this knowledge, however, pure (R)-thalidomide is not safe, because enzymes in the body rapidly interconvert the two enantiomers.
As a historical note, thalidomide was never approved for use in the United States. This was thanks in large part to the efforts of Dr. Frances Kelsey, a Food and Drug officer who, at peril to her career, blocked its approval due to her concerns about the lack of adequate safety studies, particularly with regard to the drug’s ability to enter the bloodstream of a developing fetus. Unfortunately, though, at that time clinical trials for new drugs involved widespread and unregulated distribution to doctors and their patients across the country, so families in the U.S. were not spared from the damage caused.
Very recently a close derivative of thalidomide has become legal to prescribe again in the United States, with strict safety measures enforced, for the treatment of a form of blood cancer called multiple myeloma. In Brazil, thalidomide is used in the treatment of leprosy – but despite safety measures, children are still being born with thalidomide-related defects.
Exercises
1. Determine the stereochemical configurations of the chiral centers in the biomolecules shown below.
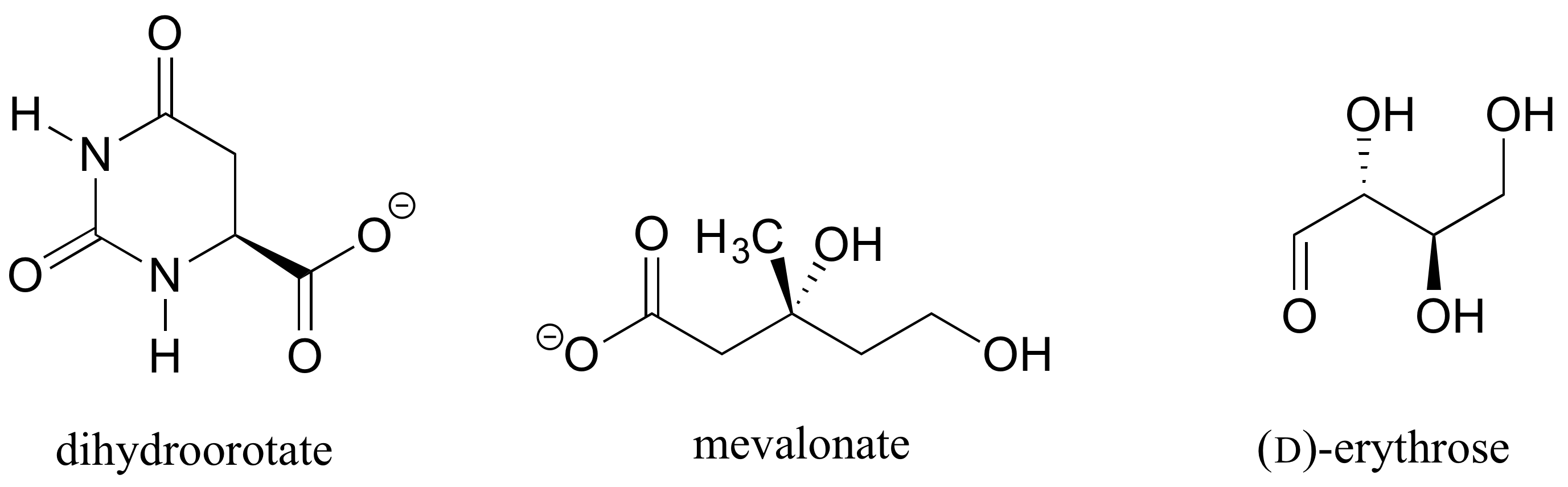
[reveal-answer q=”944882″]Show Solution[/reveal-answer]
[hidden-answer a=”944882″]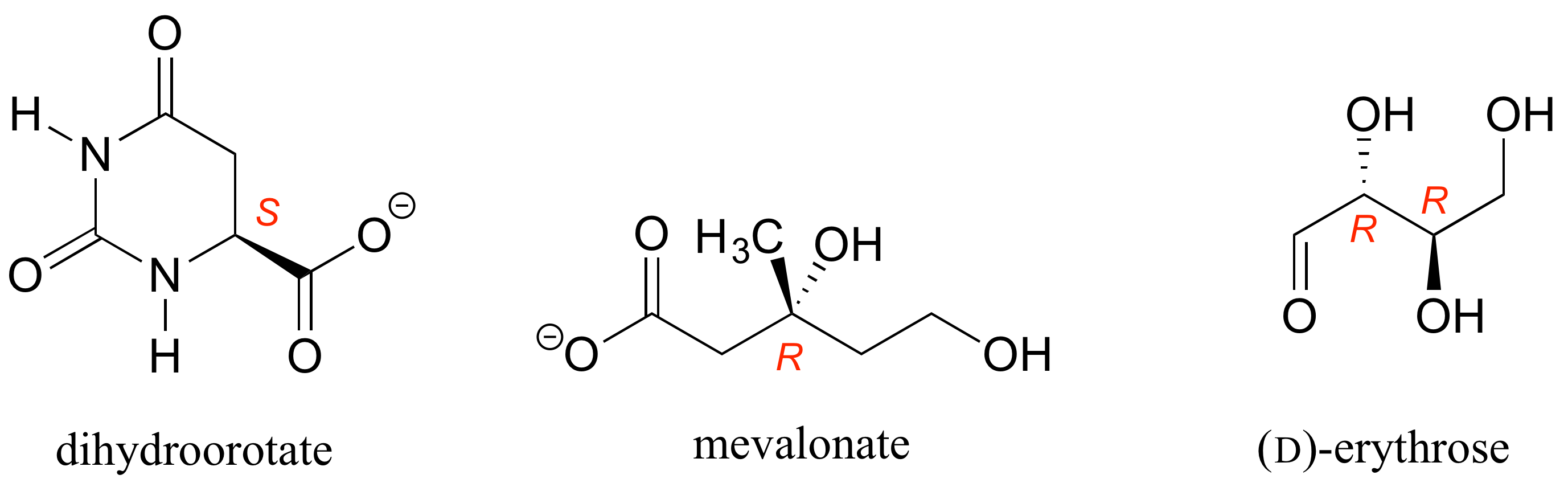 [/hidden-answer]
[/hidden-answer]
2. Should the (R) enantiomer of malate have a solid or dashed wedge for the C-O bond in the figure below?
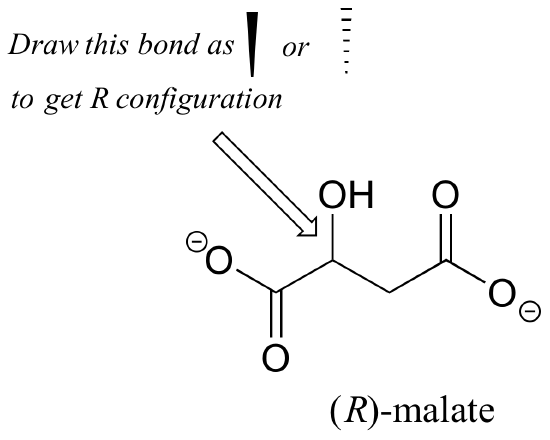
[reveal-answer q=”421115″]Show Solution[/reveal-answer]
[hidden-answer a=”421115″]
The C-O bond should be drawn as a dash to get the R configuration.
[/hidden-answer]
3. Using solid or dashed wedges to show stereochemistry, draw the (R) enantiomer of ibuprofen and the (S) enantiomer of 2-methylerythritol-4-phosphate (structures are shown earlier in this chapter without stereochemistry)
[reveal-answer q=”489949″]Show Solution[/reveal-answer]
[hidden-answer a=”489949″]
The C-O bond should be drawn as a dash to get the R configuration.
[/hidden-answer]
Khan Academy video tutorial on the R-S naming system
E,Z Notation
The configuration about double bonds is undoubtedly best specified by the cis-trans notation when there is no ambiguity involved. Unfortunately, many compounds cannot be described adequately by the cis-trans system. Consider, for example, configurational isomers of 1-fluoro-1-chloro-2-bromo-2-iodo-ethene, $$9$$ and $$10$$. There is no obvious way in which the cis-trans system can be used:
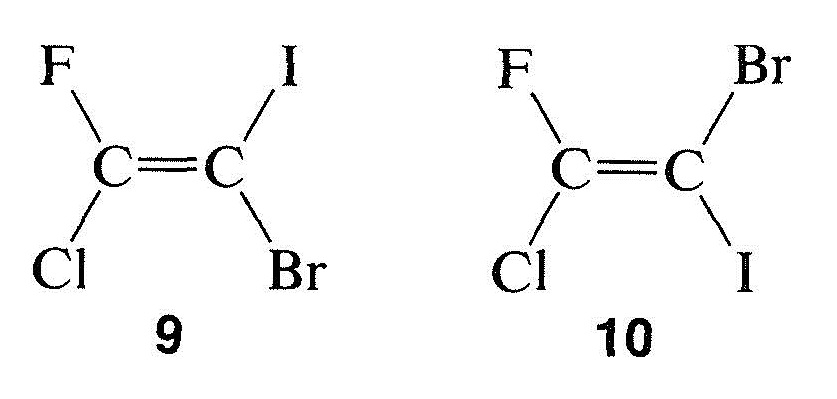
A system that is easy to use and which is based on the sequence rules already described for the $$R$$,$$S$$ system works as follows:
- An order of precedence is established for the two atoms or groups attached to each end of the double bond according to the sequence rules shown (for R/S) above. When these rules are applied to 1-fluoro-1-chloro-2-bromo-2-iodoethene, the priority sequence is:
- at carbon atom 1, $$\ce{Cl} > \ce{F}$$
- at carbon atom 2, $$\ce{I} > \ce{Br}$$
- Examination of the two configurations shows that the two priority groups – one on each end – are either on the same side of the double bond or on opposite sides:
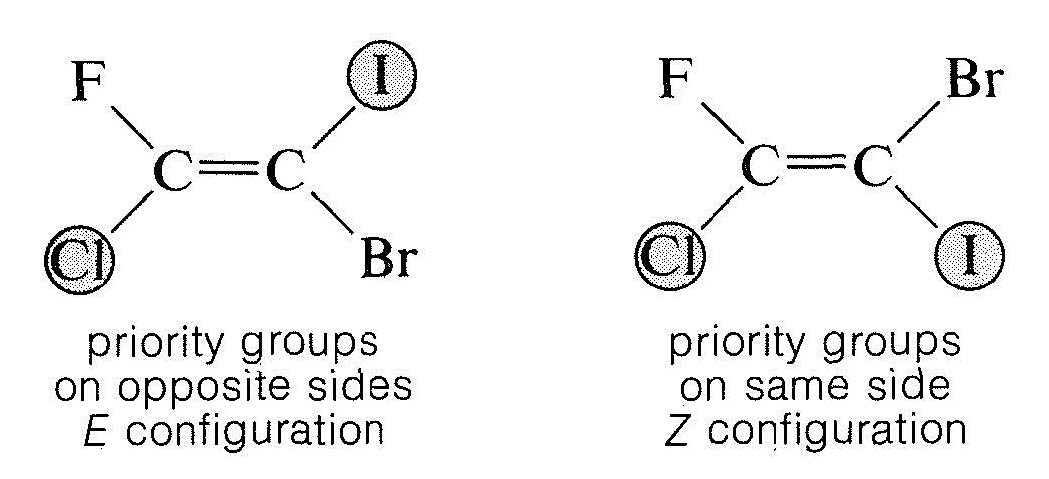
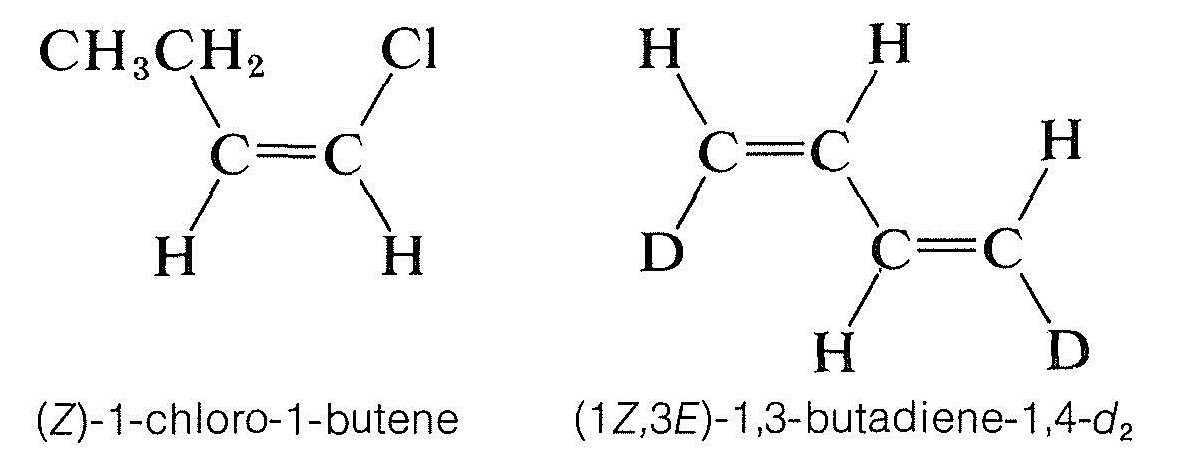
The $$Z$$ isomer is designated as the isomer in which the top priority groups are on the same side ($$Z$$ is taken from the German word zusammen – together). The $$E$$ isomer has these groups on opposite sides ($$E$$, German for entgegen – across).$$^2$$ Two further examples show how the nomenclature is used:
This system is especially useful for oximes, which have the structural feature $$\ce{-C=N-OH}$$. The two possible configurations at the double bond in the oxime of ethanal are $$11$$ and $$12$$:
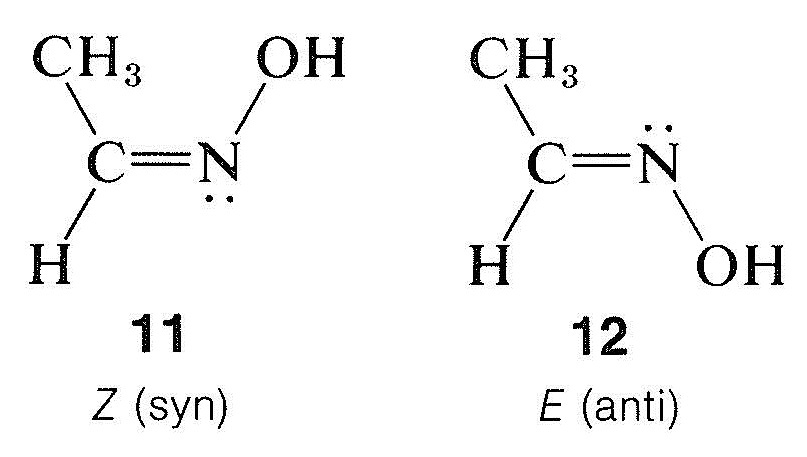
The cis-trans notation does not work well here, and structure $$11$$ has the $$Z$$ configuration and $$12$$ the $$E$$ configuration. In the older chemical literature, these stereoisomers were designated as syn and anti forms, but these names are really no better than cis and trans.
- 3.4: Naming chiral centers: the R and S system. Authored by: Tim Soderbergu00a0(University of Minnesota, Morris). Located at: https://chem.libretexts.org/Textbook_Maps/Organic_Chemistry/Book%3A_Organic_Chemistry_with_a_Biological_Emphasis_(Soderberg)/Chapter_03%3A_Conformations_and_Stereochemistry/03.4%3A_Naming_chiral_centers%3A_the_R_and_S_system. Project: Chemistry LibreTexts. License: CC BY-NC-SA: Attribution-NonCommercial-ShareAlike


