7.2: Levels of reactions
- Page ID
- 225797
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Introduction
Before we begin studying chemical reactions in depth, it is important to understand what level at which we are working. Are we focusing on the details of electron flow, to understand exactly how each bond is broken and made? Or are we planning a multi-step synthesis, as in the previous section, where we are more interested in the big picture?
If we are planning a long car journey, we face the same problem. When my family planned a long cross-country car journey in 2014 from upstate New York to San Francisco, we began by looking at the overall trip. What route should we take? Would we like to see the Grand Canyon, or take a more northerly route via Yellowstone National Park? Where should we have our overnight stays? At this high level, this is analagous to planning a multi-step synthesis, for example when a pharmaceutical R & D team maps out the synthesis of a new drug. “Should we make it via the ester, or via the ketone?” At this point the details aren’t too important, unless they are thinking about hazardous steps; they are mainly thinking about the most effective way to make the drug.
Having decided on a route (via the Badlands and Yellowstone), we began to plan the individual days’ journeys. These are like the individual reactions in our synthesis. Should we go to Sudbury, ON via Niagara Falls, or via Ottawa? When chemists plan a particular reaction, they will look at what reagents to use (considering costs, hazards), conditions (such as time, temperature), and efficiency (yield, workup, atom economy). This is at the reaction level.
Finally comes the day to leave, and we enter the details of the first day’s journey into the GPS system (SatNav). This is the lowest level we will work at. It gives us the details of exactly how we will get to Sudbury. It tells us to go north along our street for 100 yards, then to turn left, and so on. This is analagous to listing the elementary steps in the mechanism of the reaction. This can be useful for us to know when we are planning to make changes to the details – for example when we are trying to replace a toxic solvent or to optimize the yield – but the mechanism is not particularly important when planning the whole trip.
Levels of chemical reactions
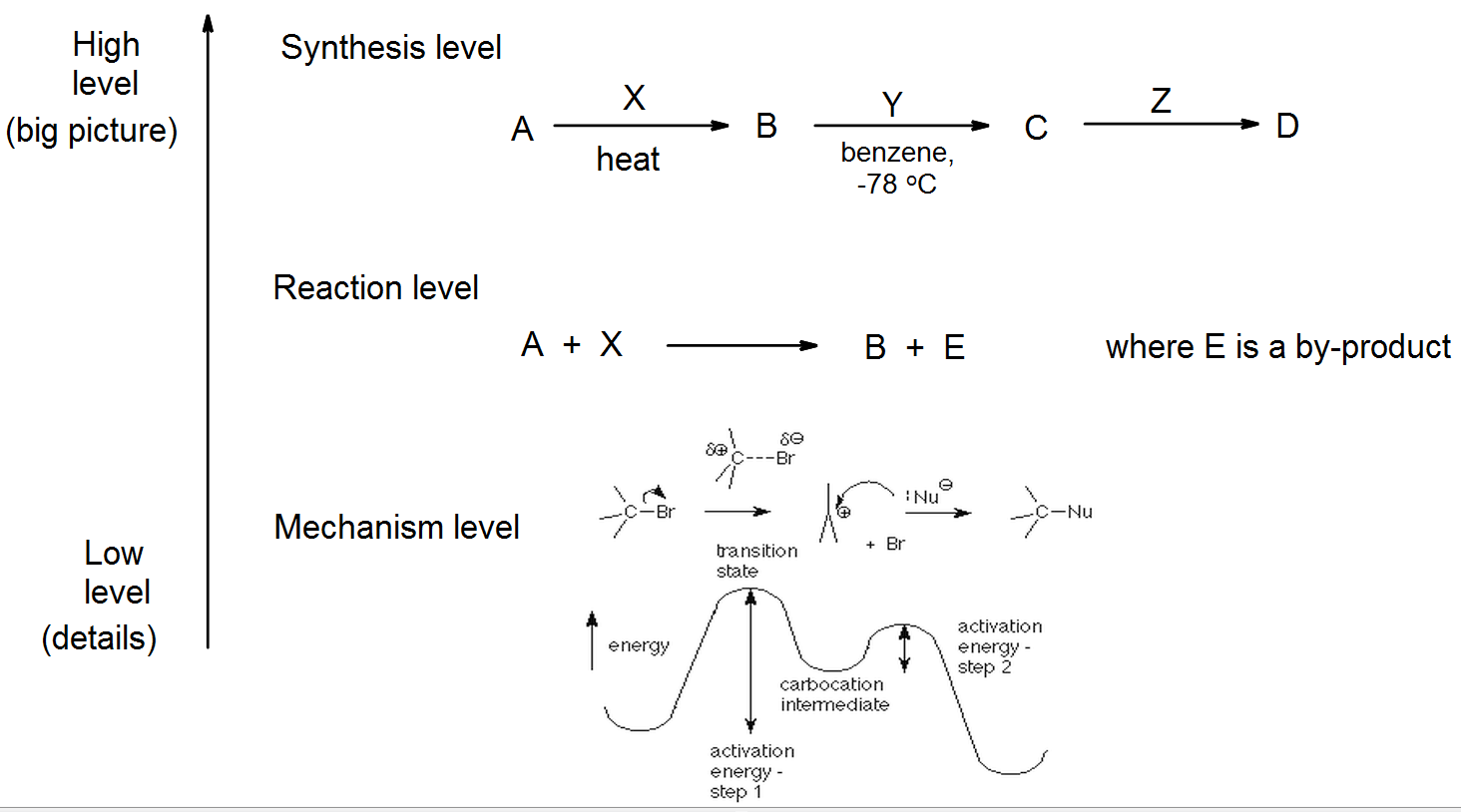
Sources of confusion
When we plan a long trip, sometimes we may use the same words (such as journey) at different levels: Do we mean the whole two week trip, or just one day? Or just the 100 yards down the street? Unfortunately, the language of organic chemistry can be very confusing for the beginner, because we may talk about electrophilic addition as one step of the mechanism of a reaction, or we may be talking about the whole process. In chapter 10 we will study processes where the overall result is an addition, driven by an electrophile; all will include one elementary step called an electrophilic addition. We will see several other cases, such as SN2 or E2 reactions, where the entire reaction takes place in one step, so the name for the elementary step is the same as for the overall reaction.
Another common term is the word “step”. As you learnt in general chemistry, an elementary step is the smallest part of a reaction mechanism – just like our GPS instruction to go 100 yards down the street. The elementary step is the basic building block for the mechanism of a reaction, and we may talk about a three step mechanism in those terms. However, when we look at the synthesis level, we may talk about the steps in the synthesis, and say that “the synthesis of ibuprofen involves four steps.” This is like saying “our trip to San Francisco has twelve steps (twelve day journeys).” I will try to refer to these as synthetic steps. If you hear the word “step,” use the context to understand exactly what is meant – is it an elementary step in a mechanism, or a synthetic step that forms part of a synthesis?
A similar problem occurs with the word “intermediate.” As we saw in chapter 5, we frequently refer to an intermediate that occurs in a mechanism, and in section 5.6. we looked at some common intermediates such as carbocations. But again, in a multi-step synthesis we may have isolated products which we refer to as intermediates, equivalent to the motels or campsites where we end each day’s journey. I will try to distinguish between these by referring to reaction intermediates or synthetic intermediates, especially in the latter case, but often you will need to use the context to work out the meaning.
This blurring in the wording between the levels can be confusing, so make sure you understand at what level the term is being used. Once you are comfortable with this, the wording will become less important as you begin to focus on the concepts behind the words.
Another analogy: writing sentences
When you learn organic chemistry for the first time, it can seem overwhelming, as almost nothing is familiar. This is similar to how some first graders feel when faced with the challenging task of learning to read.
In this analogy, reactions are like words. When we break down a reaction to study the details of electron flow, this is like looking at the spelling, i.e., the exact sequence of letters (= elementary steps) used to make a word (= reaction). This is important for to learn. But if we only ever learn to spell words, and never use them, we are missing out on a lot! Language comes to life when we learn to put words together to make sentences. Likewise, organic reactions come to life when we learn to put sequences together to make a multi-step synthesis. When we read, our brains often tune out the details of the spelling and focus on the overall meaning behind the sentence. In the same way, when we work on the “big picture” of a long synthesis, we need to focus on the whole sentence. Nevertheless, we must not ignore the spelling, and if we are writing poetry, the order of sounds remains important.
Some writers, such as William Shakespeare, are able to craft wonderful combinations of words that are truly elegant and beautiful. Likewise, organic chemists often talk about a synthesis in similar terms, and they mean that the “elegant” synthesis has a wonderful flow and efficiency in its design. The chemist Robert Burns Woodward is regarded as one of the greatest synthetic chemists of all time, and many today would see him as a great “poet” of organic chemistry.
- Authored by: Martin Walker. Provided by: SUNY Potsdam. Located at: http://directory.potsdam.edu/?function=user=walkerma. License: CC BY-SA: Attribution-ShareAlike
- Reaction coordinate diagram for SN1. Authored by: Kirk McMichael . Provided by: Washington State University. Located at: https://chem.libretexts.org/Textbook_Maps/Organic_Chemistry/Book%3A_Organic_Chemistry_-_A_%22Carbonyl_Early%22_Approach_(McMichael)/25%3A_Elimination_-_E2_and_E1. Project: Organic Chemistry - A Carbonyl Early Approach. License: CC BY-NC: Attribution-NonCommercial

