20.1: Introduction to polar pi Bonds
- Page ID
- 225887
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Nucleophilic additions to aldehydes and ketones: the general picture
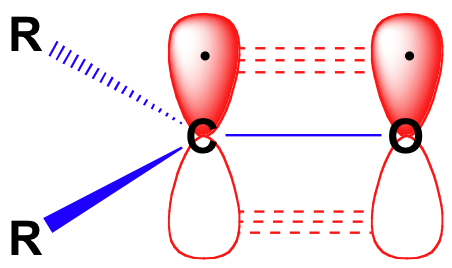 The carbon-oxygen double bond is polar: oxygen is more electronegative than carbon, so electron density is higher on the oxygen side of the bond and lower on the carbon side. Recall that bond polarity can be depicted with a dipole arrow, or by showing the oxygen as holding a partial negative charge and the carbonyl carbon a partial positive charge.
The carbon-oxygen double bond is polar: oxygen is more electronegative than carbon, so electron density is higher on the oxygen side of the bond and lower on the carbon side. Recall that bond polarity can be depicted with a dipole arrow, or by showing the oxygen as holding a partial negative charge and the carbonyl carbon a partial positive charge.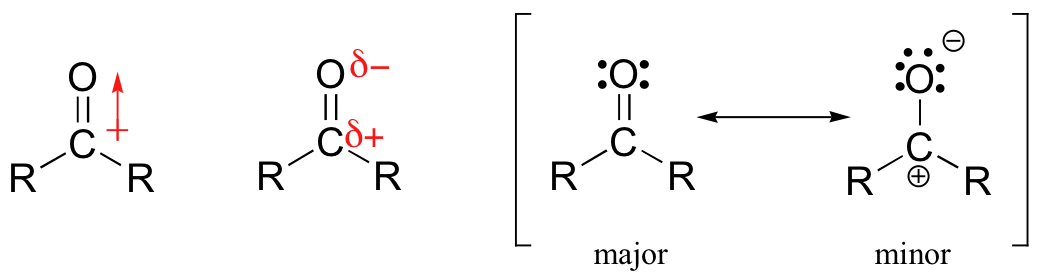 A third way to illustrate the carbon-oxygen dipole is to consider the two main resonance contributors of a carbonyl group: the major form, which is what you typically see drawn in Lewis structures, and a minor but very important contributor in which both electrons in the pbond are localized on the oxygen, giving it a full negative charge. The latter depiction shows the carbon with an empty 2p orbital and a full positive charge.The result of carbonyl bond polarization, however it is depicted, is straightforward to predict. The carbon, because it is electron-poor, is an electrophile: it is a great target for attack by an electron-rich nucleophilic group. Because the oxygen end of the carbonyl double bond bears a partial negative charge, anything that can help to stabilize this charge by accepting some of the electron density will increase the bond’s polarity and make the carbon more electrophilic. Very often a general acid group serves this purpose, donating a proton to the carbonyl oxygen.
A third way to illustrate the carbon-oxygen dipole is to consider the two main resonance contributors of a carbonyl group: the major form, which is what you typically see drawn in Lewis structures, and a minor but very important contributor in which both electrons in the pbond are localized on the oxygen, giving it a full negative charge. The latter depiction shows the carbon with an empty 2p orbital and a full positive charge.The result of carbonyl bond polarization, however it is depicted, is straightforward to predict. The carbon, because it is electron-poor, is an electrophile: it is a great target for attack by an electron-rich nucleophilic group. Because the oxygen end of the carbonyl double bond bears a partial negative charge, anything that can help to stabilize this charge by accepting some of the electron density will increase the bond’s polarity and make the carbon more electrophilic. Very often a general acid group serves this purpose, donating a proton to the carbonyl oxygen. The same effect can also be achieved if a Lewis acid, such as a magnesium ion, is located near the carbonyl oxygen.Unlike the situation in a nucleophilic substitution reaction, when a nucleophile attacks an aldehyde or ketone carbon there is no leaving group – the incoming nucleophile simply ‘pushes’ the electrons in the pi bond up to the oxygen.
The same effect can also be achieved if a Lewis acid, such as a magnesium ion, is located near the carbonyl oxygen.Unlike the situation in a nucleophilic substitution reaction, when a nucleophile attacks an aldehyde or ketone carbon there is no leaving group – the incoming nucleophile simply ‘pushes’ the electrons in the pi bond up to the oxygen.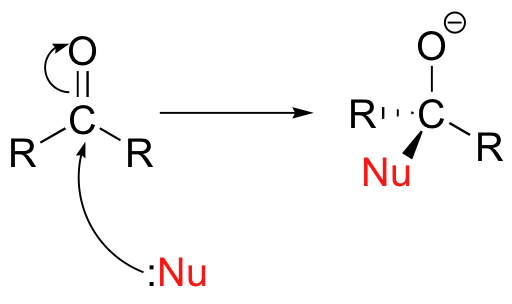 Alternatively, if you start with the minor resonance contributor, you can picture this as an attack by a nucleophile on a carbocation.
Alternatively, if you start with the minor resonance contributor, you can picture this as an attack by a nucleophile on a carbocation. 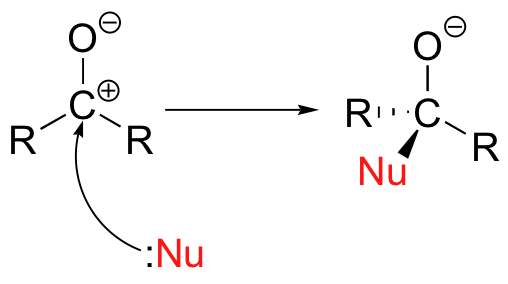
After the carbonyl is attacked by the nucleophile, the negatively charged oxygen has the capacity to act as a nucleophile. However, most commonly the oxygen acts instead as a base, abstracting a proton from a nearby acid group in the solvent or enzyme active site.

This very common type of reaction is called a nucleophilic addition. In many biologically relevant examples of nucleophilic addition to carbonyls, the nucleophile is an alcohol oxygen or an amine nitrogen, or occasionally a thiol sulfur. In one very important reaction type known as an aldol reaction (which we will learn about in section 13.3) the nucleophile attacking the carbonyl is a resonance-stabilized carbanion. In this chapter, we will concentrate on reactions where the nucleophile is an oxygen or nitrogen.
. . . on to the next section . . .
Contributors
- Organic Chemistry With a Biological Emphasis by Tim Soderberg (University of Minnesota, Morris)
Carbonyl group: Mechanisms of addition
Ionic Addition to Carbonyl Group
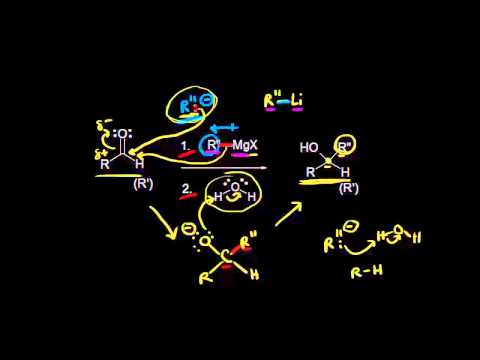
As a result of the dipole shown in the resonance structures, polar reagents such as LiAlH4 and NaBH4 (hydride reagents) or R’MgX (Grignard reagent) will reduce the carbonyl groups, and ultimately convert unsaturated aldehydes and ketones into unsaturated alcohols. Since these reagents are extremely basic, their addition reactions are irreversible.
There are, however, addition reactions with less basic nucleophiles such as water, thiols, and amines that are capable of establishing equilibria or reversible reactions. These less basic reagents can react with the carbonyl group via two pathways: nucleophilic addition-protonation and electrophilic addition-protonation.
Addition of strong nucleophiles: Nucleophilic addition-protonation
With strong nucleophiles, direct nucleophilic attack of the electrophilic carbon takes place. As the nucleophile approaches the electrophilic carbon, two valence electrons from the nucleophile form a covalent bond to the carbon. As this occurs, the electron pair from the pi bond transfers completely over to the oxygen which produces the intermediate alkoxide ion. This alkoxide ion, with a negative charge on oxygen is susceptible to protonation from a protic solvent like water or alcohol, giving the final addition reaction.
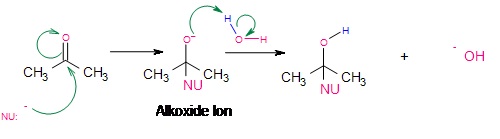
Acid-catalyzed nucleophilic addition of weak nucleophiles.
Under acidic conditions, protonation of the carbonyl oxygen takes place. Then nucleophilic attack by the nucleophile finishes the addition reaction. This type of reaction works best when the reagent being used is a very mildly basic nucleophile.
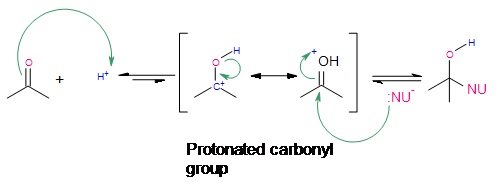
Outside links
References
- Vollhardt, K. P.C. & Shore, N. (2007). Organic Chemistry (5th Ed.). New York: W. H. Freeman. p. 775-777
- Otera, Junzo, ed. Modern Carbonyl Chemistry. Weinheim; Chichester: Wiley-VCH, 2000.
Relative Reactivity of Carbonyl Compounds to Nucleophilic Addition
In general aldehydes are more reactive than ketones because of the lack of stabilizing alkyl groups. The primary carbocation formed in the in the polarizing resonance structure of an aldehyde (discussed above) is less stable and therefore more reactive than the secondary carbocation formed by a ketone.
Contributors
- Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
- Prof. Steven Farmer (Sonoma State University)
Stereochemistry of the nucleophilic addition reaction

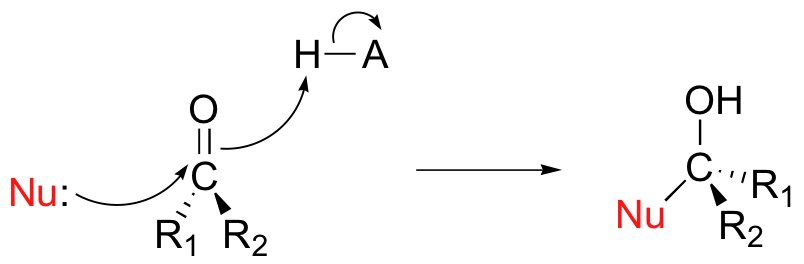
If the reaction is catalyzed by an enzyme, the stereochemistry of addition is tightly controlled, and leads to one specific stereoisomer – this is because the nucleophilic and electrophilic substrates are bound in a specific positions within the active site, so that attack must occur specifically from one side. If, however, the reaction occurs uncatalyzed in solution, then either side of the carbonyl is equally likely to be attacked, and the result will be a 50:50 racemic mixture.
Contributors
- Organic Chemistry With a Biological Emphasis by Tim Soderberg (University of Minnesota, Morris)
Videos


- Organic Chemistry With a Biological Emphasis . Authored by: Tim Soderberg. Located at: https://chem.libretexts.org/Textbook_Maps/Organic_Chemistry/Book%3A_Organic_Chemistry_with_a_Biological_Emphasis_(Soderberg)/11%3A_Nucleophilic_carbonyl_addition_reactions/11.1%3A_Nucleophilic_additions_to_aldehydes_and_ketones%3A_the_general_picture. Project: Chemistry LibreTexts. License: CC BY-NC-SA: Attribution-NonCommercial-ShareAlike
- Carbonyl Group-Mechanisms of Addition. Authored by: K. P.C. Vollhardt, N. Shore, Junzo Otera. Located at: https://chem.libretexts.org/?title=Textbook_Maps/Organic_Chemistry/Supplemental_Modules_(Organic_Chemistry)/Aldehydes_and_Ketones/Reactivity_of_Aldehydes_%26_Ketones/Carbonyl_Group-Mechanisms_of_Addition. Project: Chemistry LibreTexts. License: CC BY-NC-SA: Attribution-NonCommercial-ShareAlike
- 19.4 Nucleophilic Addition Reactions of Aldehydes and Ketones. Authored by: Dr. Dietmar Kennepohl, Prof. Steven Farmer. Located at: https://chem.libretexts.org/Textbook_Maps/Organic_Chemistry/Map%3A_Organic_Chemistry_(McMurry)/Chapter_19%3A_Aldehydes_and_Ketones%3A_Nucleophilic_Addition_Reactions/19.04_Nucleophilic_Addition_Reactions_of_Aldehydes_and_Ketones. Project: Chemistry LibreText. License: CC BY-NC-SA: Attribution-NonCommercial-ShareAlike