16.1: Oxidation of Alcohols
- Page ID
- 23558
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)This page looks at the oxidation of alcohols using acidified sodium or potassium dichromate(VI) solution. This reaction is used to make aldehydes, ketones and carboxylic acids, and as a way of distinguishing between primary, secondary and tertiary alcohols.
Oxidizing the different types of alcohols
The oxidizing agent used in these reactions is normally a solution of sodium or potassium dichromate(VI) acidified with dilute sulfuric acid. If oxidation occurs, then the orange solution containing the dichromate(VI) ions is reduced to a green solution containing chromium(III) ions. The electron-half-equation for this reaction is as follows:
\[ Cr_2O_7^{2-} + 14H^+ + 6e^- \rightarrow 2Cr^{3+} + 7H_2O\]
Primary alcohols
Primary alcohols can be oxidized to either aldehydes or carboxylic acids, depending on the reaction conditions. In the case of the formation of carboxylic acids, the alcohol is first oxidized to an aldehyde, which is then oxidized further to the acid.
An aldehyde is obtained if an excess amount of the alcohol is used, and the aldehyde is distilled off as soon as it forms. An excess of the alcohol means that there is not enough oxidizing agent present to carry out the second stage, and removing the aldehyde as soon as it is formed means that it is not present to be oxidized anyway!
If you used ethanol as a typical primary alcohol, you would produce the aldehyde ethanal, \(CH_3CHO\). The full equation for this reaction is fairly complicated, and you need to understand the electron-half-equations in order to work it out.
\[ 3CH_3CH_2OH + Cr_2O_7^{2-} + 8H^+ \rightarrow 3CH_3CHO + 2Cr^{3+} + 7H_2O\]
In organic chemistry, simplified versions are often used that concentrate on what is happening to the organic substances. To do that, oxygen from an oxidizing agent is represented as \([O]\). That would produce the much simpler equation:


It also helps in remembering what happens. You can draw simple structures to show the relationship between the primary alcohol and the aldehyde formed.

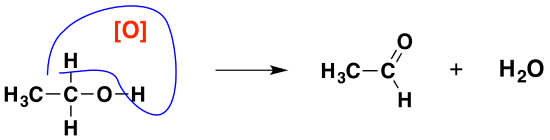
Full oxidation to carboxylic acids
An excess of the oxidizing agent must be used, and the aldehyde formed as the half-way product should remain in the mixture. The alcohol is heated under reflux with an excess of the oxidizing agent. When the reaction is complete, the carboxylic acid is distilled off. The full equation for the oxidation of ethanol to ethanoic acid is as follows:
\[ 3CH_3CH_2OH + 2Cr_2O_7^{2-} + 16H+ \rightarrow 3CH_3COOH + 4Cr^{3+} + 11H_2O\]
The more typical simplified version looks like this:
\[ CH_3CH_2OH + 2[O] \rightarrow CH_3COOH + H_2O\]
Alternatively, you could write separate equations for the two stages of the reaction - the formation of ethanal and then its subsequent oxidation.
\[ CH_3CH_2OH + [O] \rightarrow CH_3CHO + H_2O\]
\[ CH_3CHO + [O] \rightarrow CH_3COOH\]
This is what is happening in the second stage:


Secondary alcohols
Secondary alcohols are oxidized to ketones - and that's it. For example, if you heat the secondary alcohol propan-2-ol with sodium or potassium dichromate(VI) solution acidified with dilute sulfuric acid, propanone is formed. Changing the reaction conditions makes no difference to the product. Folloiwng is the simple version of the equation, showing the relationship between the structures:


If you look back at the second stage of the primary alcohol reaction, you will see that an oxygen inserted between the carbon and the hydrogen in the aldehyde group to produce the carboxylic acid. In this case, there is no such hydrogen - and the reaction has nowhere further to go.
Tertiary alcohols
Tertiary alcohols are not oxidized by acidified sodium or potassium dichromate(VI) solution - there is no reaction whatsoever. If you look at what is happening with primary and secondary alcohols, you will see that the oxidizing agent is removing the hydrogen from the -OH group, and a hydrogen from the carbon atom is attached to the -OH. Tertiary alcohols don't have a hydrogen atom attached to that carbon.
You need to be able to remove those two particular hydrogen atoms in order to set up the carbon-oxygen double bond.
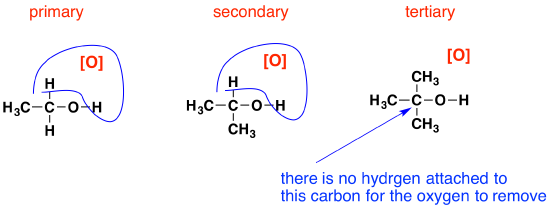
Using these reactions as a test for the different types of alcohols
First, the presence of an alcohol must be confirmed by testing for the -OH group. The liquid would need to be verified as neutral, free of water and that it reacted with solid phosphorus(V) chloride to produce a burst of acidic steamy hydrogen chloride fumes. A few drops of the alcohol would be added to a test tube containing potassium dichromate(VI) solution acidified with dilute sulfuric acid. The tube would be warmed in a hot water bath.
Determining the tertiary alcohol
In the case of a primary or secondary alcohol, the orange solution turns green. With a tertiary alcohol, there is no color change. After heating, the following colors are observed:
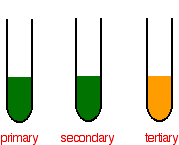
Distinguishing between the primary and secondary alcohols
A sufficient amount of the aldehyde (from oxidation of a primary alcohol) or ketone (from a secondary alcohol) must be produced to be able to test them. There are various reactions that aldehydes undergo that ketones do not. These include the reactions with Tollens' reagent, Fehling's solution and Benedict's solution, and these reactions are covered on a separate page.
These tests can be difficult to carry out, and the results are not always as clear-cut as the books say. A much simpler but fairly reliable test is to use Schiff's reagent. Schiff's reagent is a fuchsin dye decolorized by passing sulfur dioxide through it. In the presence of even small amounts of an aldehyde, it turns bright magenta.
It must, however, be used absolutely cold, because ketones react with it very slowly to give the same color. If you heat it, obviously the change is faster - and potentially confusing. While you are warming the reaction mixture in the hot water bath, you can pass any vapors produced through some Schiff's reagent.
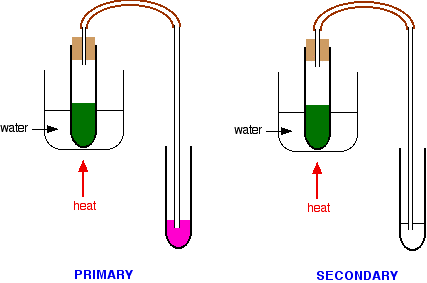
- If the Schiff's reagent quickly becomes magenta, then you are producing an aldehyde from a primary alcohol.
- If there is no color change in the Schiff's reagent, or only a trace of pink color within a minute or so, then you are not producing an aldehyde; therefore, no primary alcohol is present.
Because of the color change to the acidified potassium dichromate(VI) solution, you must, therefore, have a secondary alcohol. You should check the result as soon as the potassium dichromate(VI) solution turns green - if you leave it too long, the Schiff's reagent might start to change color in the secondary alcohol case as well.
Contributors
Jim Clark (Chemguide.co.uk)
Further Reading
Wikipedia
Khan Academy
Carey 5th Ed Online
Chemtube3D
MasterOrganicChemistry

