16.3: Reversible Nucleophilic Addition
- Page ID
- 375444
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)So far, we have discussed the first of two primary ways for nucleophilic additions to carbonyls – irreversible reactions. Another way of classifying nucleophilic addition reactions is to base the classification on the reaction conditions: basic or acidic.
A. Basic conditions: Under these conditions, deprotonation of a nucleophile raises the HOMO, making it more reactive. Nucleophilic addition occurs to give a tetrahedral intermediate which then either 1) protonates immediately, or 2) reverses if the pKa < 15 and then protonates if given enough energy (NOTE: the reverse reaction is the exact opposite set of arrows, according to the law of microscopic reversibility). An example of case #1 is the reduction of ketones/aldehydes under kinetic control. An example of case #2 is the thermodynamic addition to the carbonyl of cyanide anion to the carbonyl.
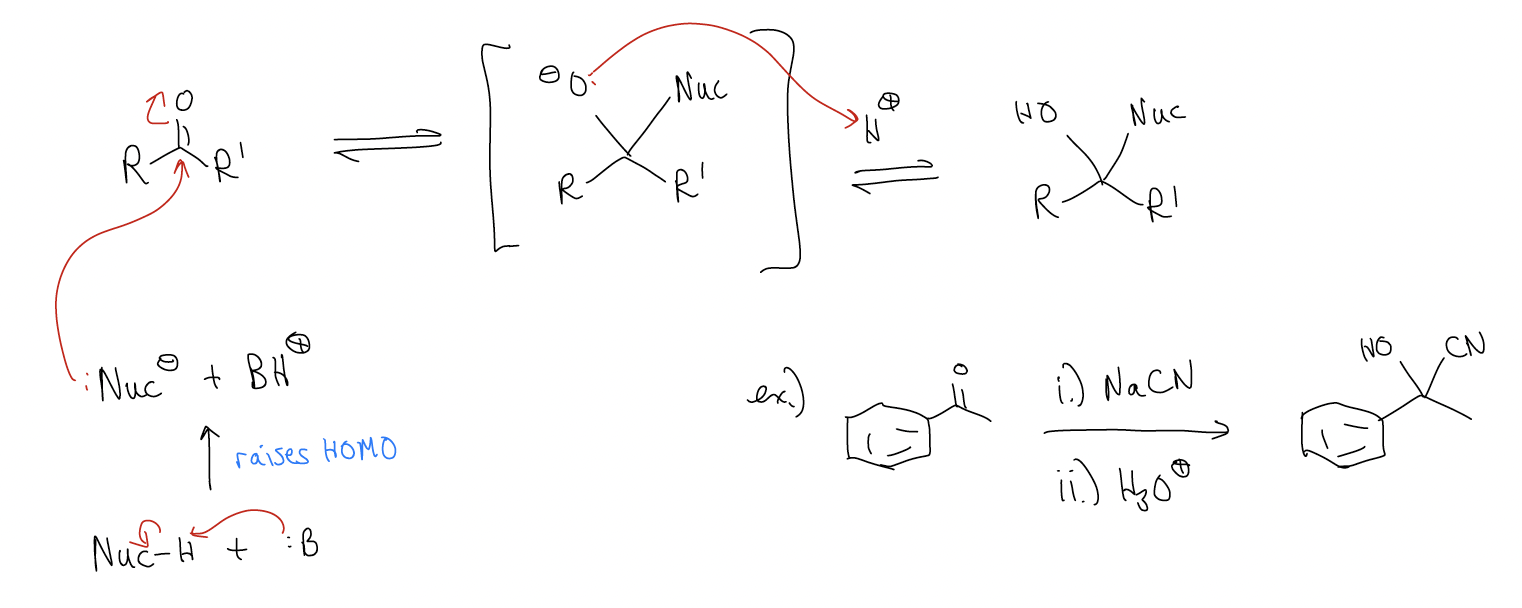
B. Acidic conditions: Under these conditions, protonation of the carbonyl (via the oxygen lone pairs) will lower the LUMO enough that a nucleophile can add. There are several more examples of this: 1) cyanohydrin formation; 2) gem-diol formation; and 3) acetal formation.
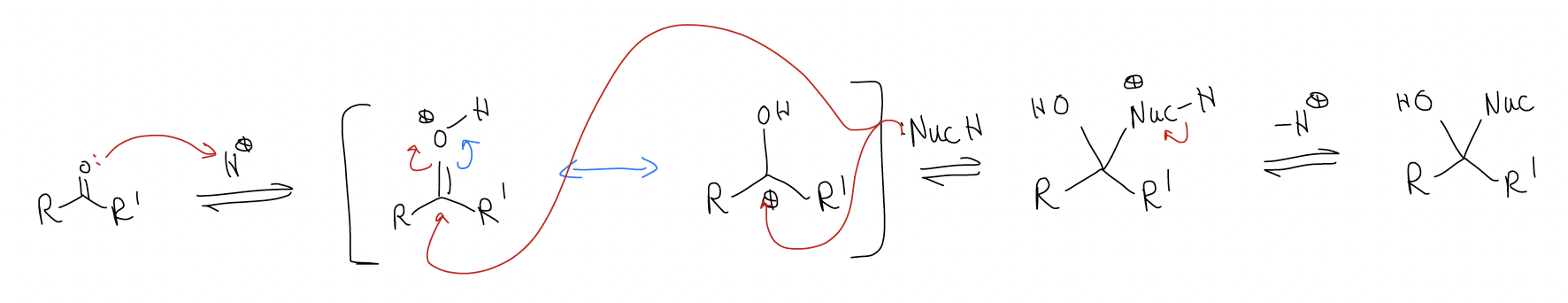
1. Cyanohydrin formation
mechanism:
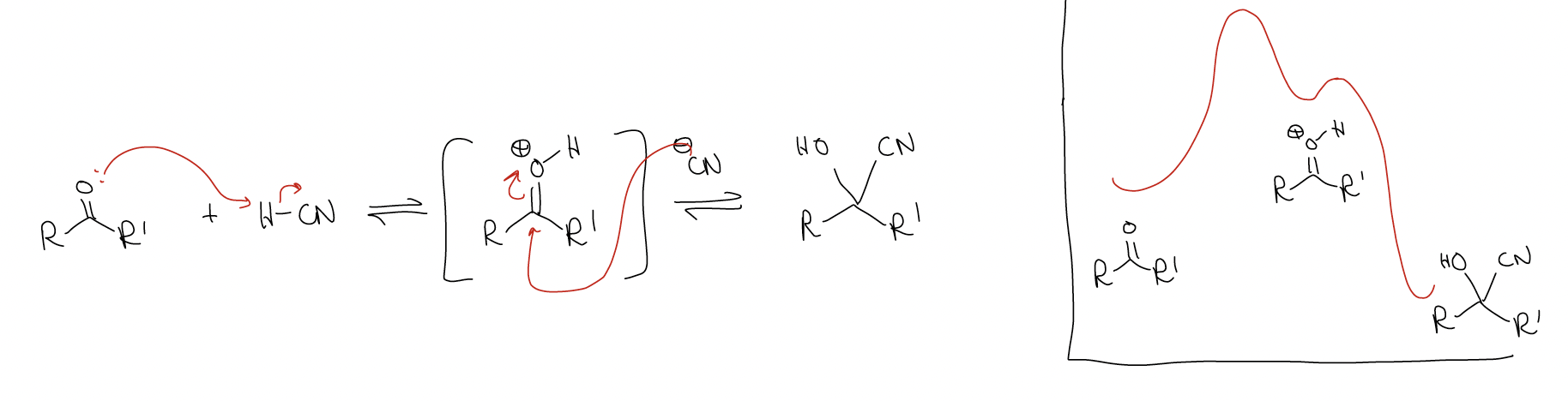
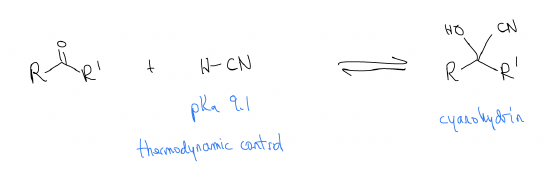
Since this reaction is reversible, there is an equilibrium constant associated with it. Consider the following two reactions:
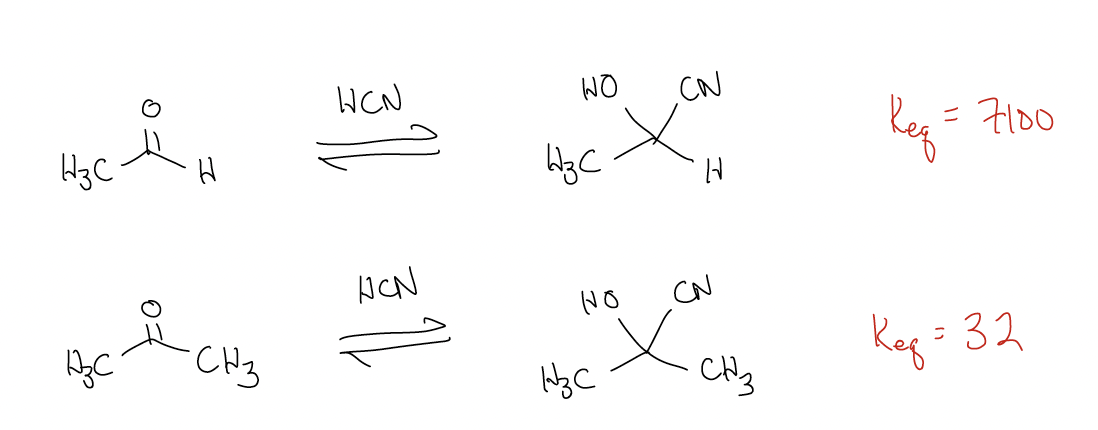
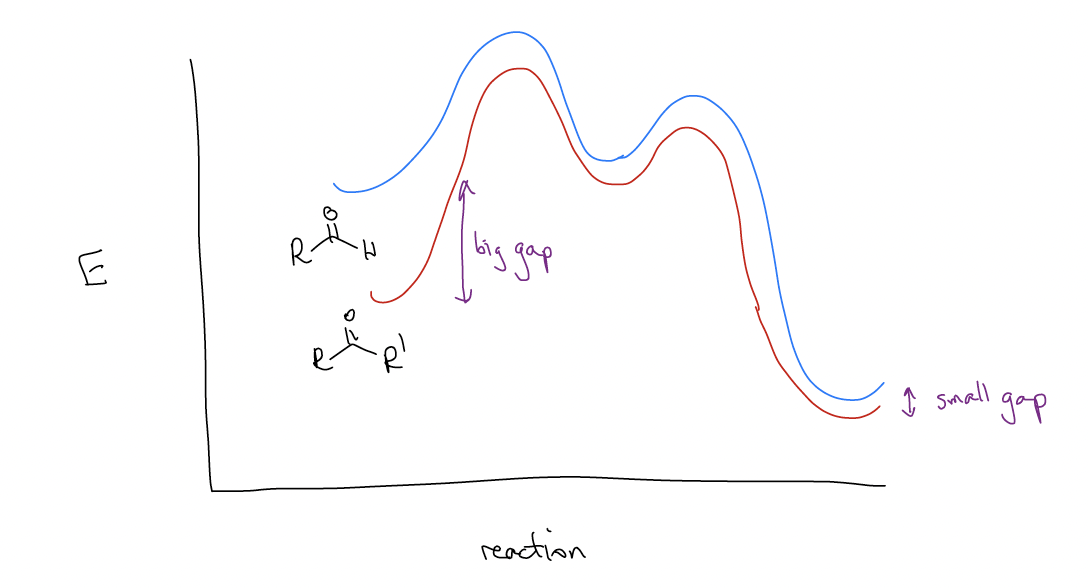
Why is there such a difference? Like most things, there is an electronic (rather than steric) explanation. The difference in the stability of the products is roughly the same, so it must arise from the stability of the starting materials. Ketones are more stable than aldehydes and since the energy of the transition states and the energies of the products are about equal, there are large differences in reactivity. We don’t care how large the activation energy (Ea) is, nor how high the transition state is, because we are under thermodynamic control – there is enough energy to overcome any energy barrier.
You might be wondering why ketones are more stable than aldehydes. This is due to \(σ\)-donation. Electrons like to be spread out and this can occur more easily in ketones via \(σ\)-donation of \(σ\)C-H into \(π^{*}\)C-O. This delocalization occurs six times, which raises the LUMO (\(π^{*}\)) of the ketone, making it less reactive.
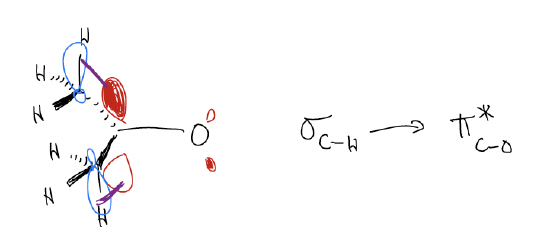
What other factors influence Keq? Consider the following data for cyanohydrin formation:
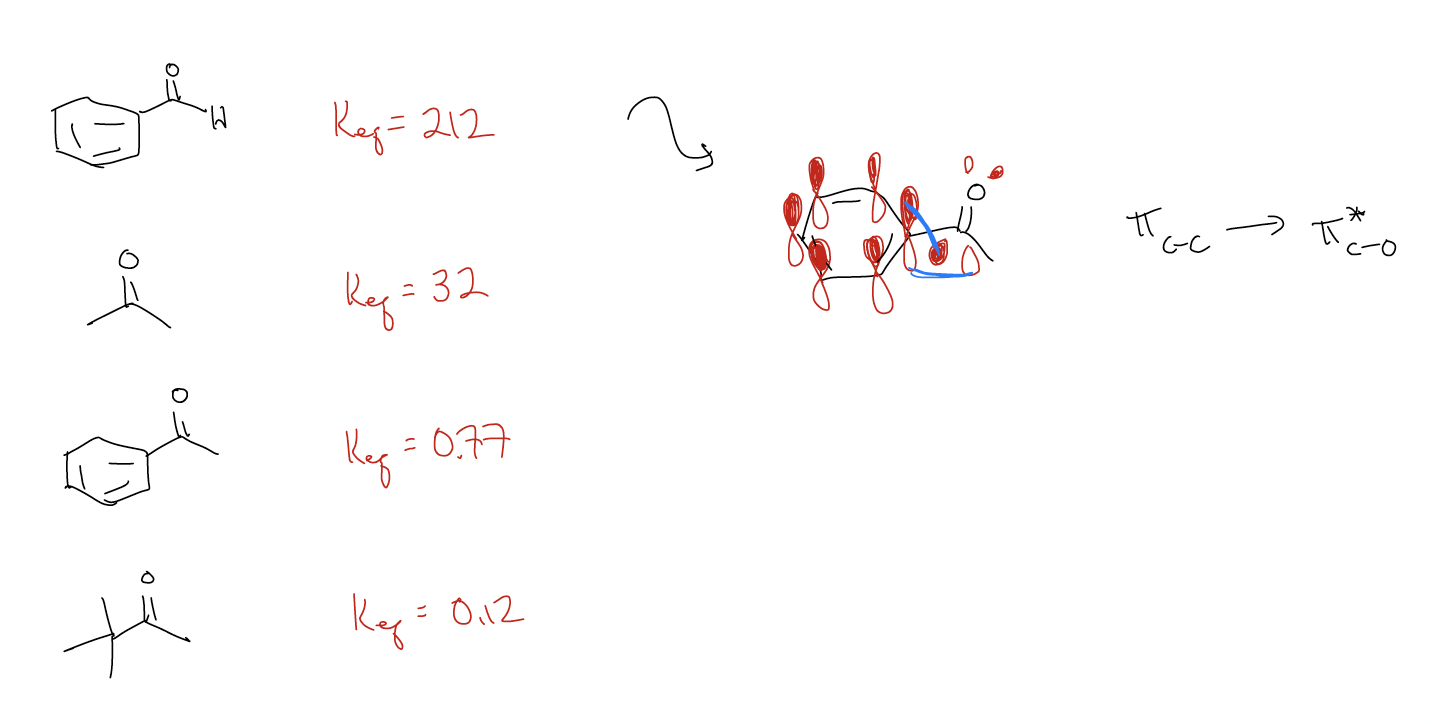
The difference between acetone and acetophenone tells us that conjugation is more powerful than \(σ\)-donation – declocalization from \(π\)C-C into \(π^{*}\)C-O occurs because these orbitals are closer in energy (better mixing). In the case of tert-butyl methyl ketone, even though the \(σ\)C-C is a better donor than \(σ\)C-H due to better orbital overlap, there is steric crowding in the transition state, so the Keq is lower.
2. Gem-diol formation – geminal means “same carbon”
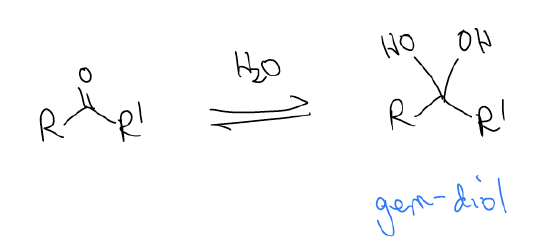
This is a very slow reaction that can be accelerated by base/acid catalysis (heat will favor the reverse direction because entropy favor bimolecular set of products). There are similar effects on Keq:
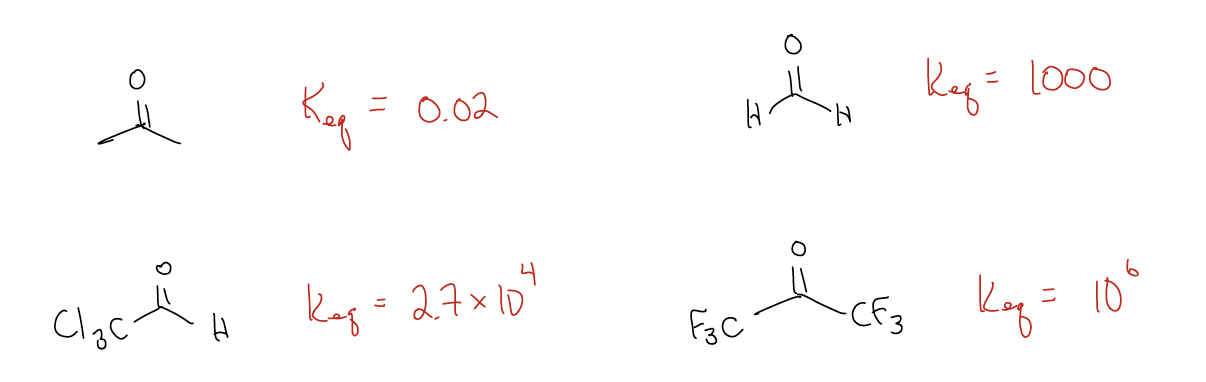
NOTE: one cannot buy formaldehyde itself, but you can access it in the following forms: 1) 40% solution in H2O; 2) para-formaldehyde and 3) s-trioxane
- base catalysis: Water is not a good nucleophile to attack the \(π^{*}\)C-O orbital. Its HOMO (nO) is too low, but we can raise this HOMO by deprotonation with base (K2CO3, NaOH, Et3N, etc.)
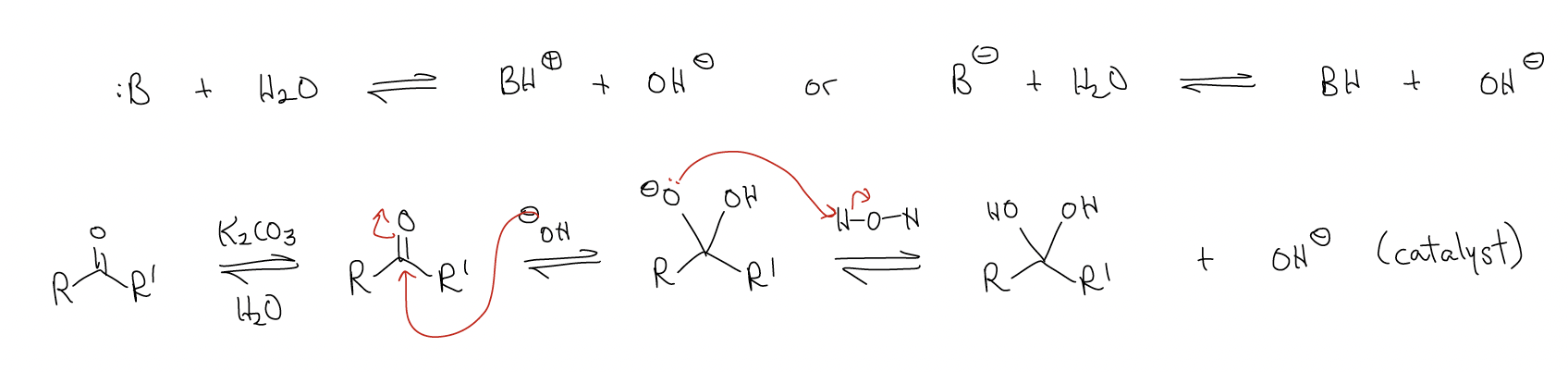
- acid catalysis: The ketone/aldehyde is not a good electrophile to react with nO of the incoming nucleophile. Its LUMO (\(π^{*}\)C-O) is too low, but we can lower the LUMO by protonation with a Bronsted acid or Lewis acid.
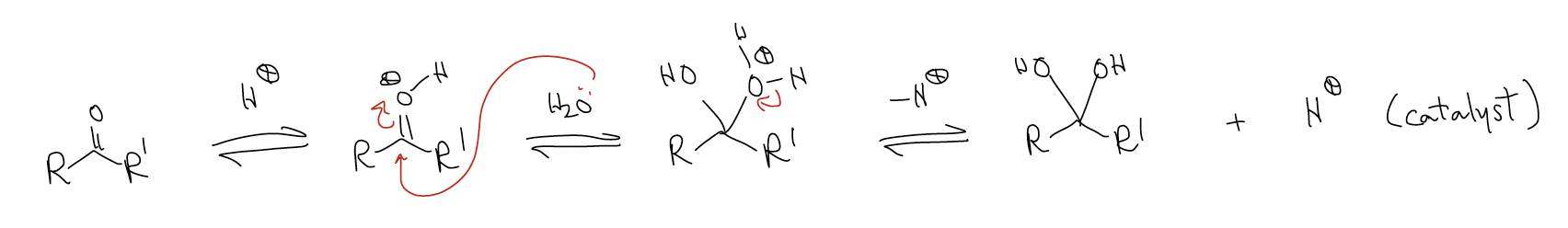
Consider the following reaction in which 16O-labeled acetone is reacted with 18O-labeled water:
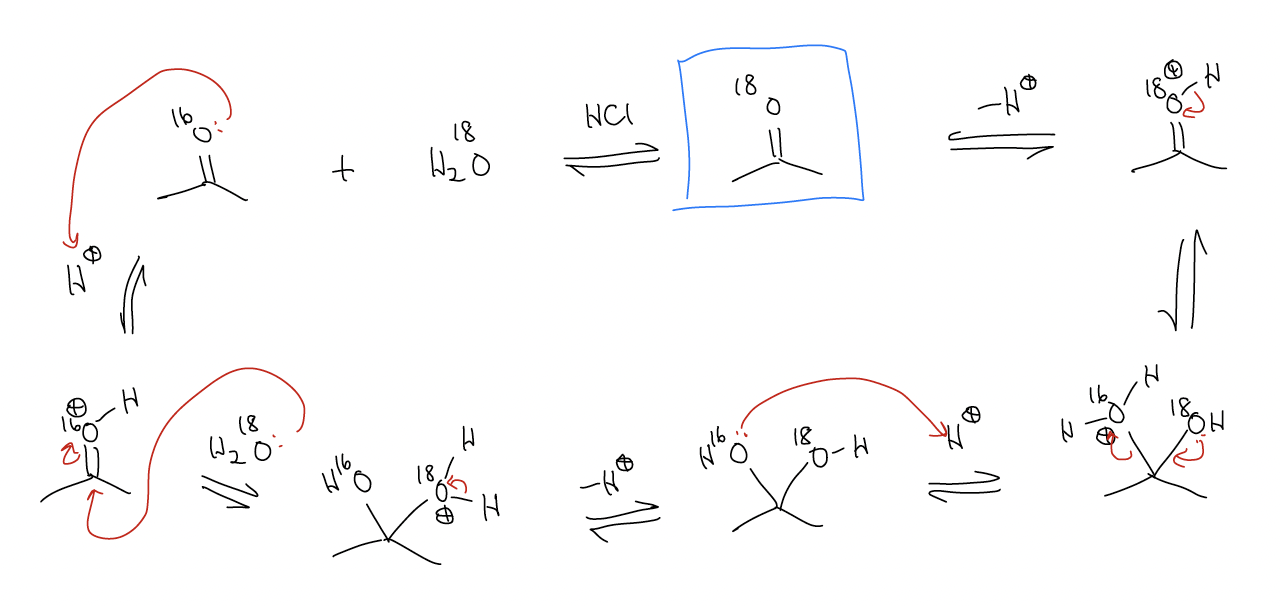
The law of microscopic reversibility states that the last three steps are just the reverse of the first three. The small amount of H216O generated will never be able to compete with H218O as solvent, so this reaction is pushed toward product.

