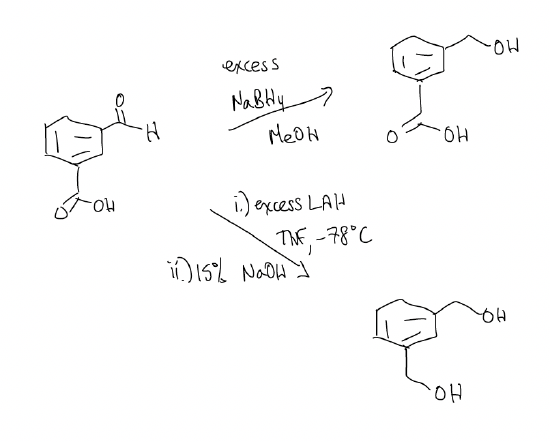
16.2: Irreversible Nucleophilic Addition
- Page ID
- 375443
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
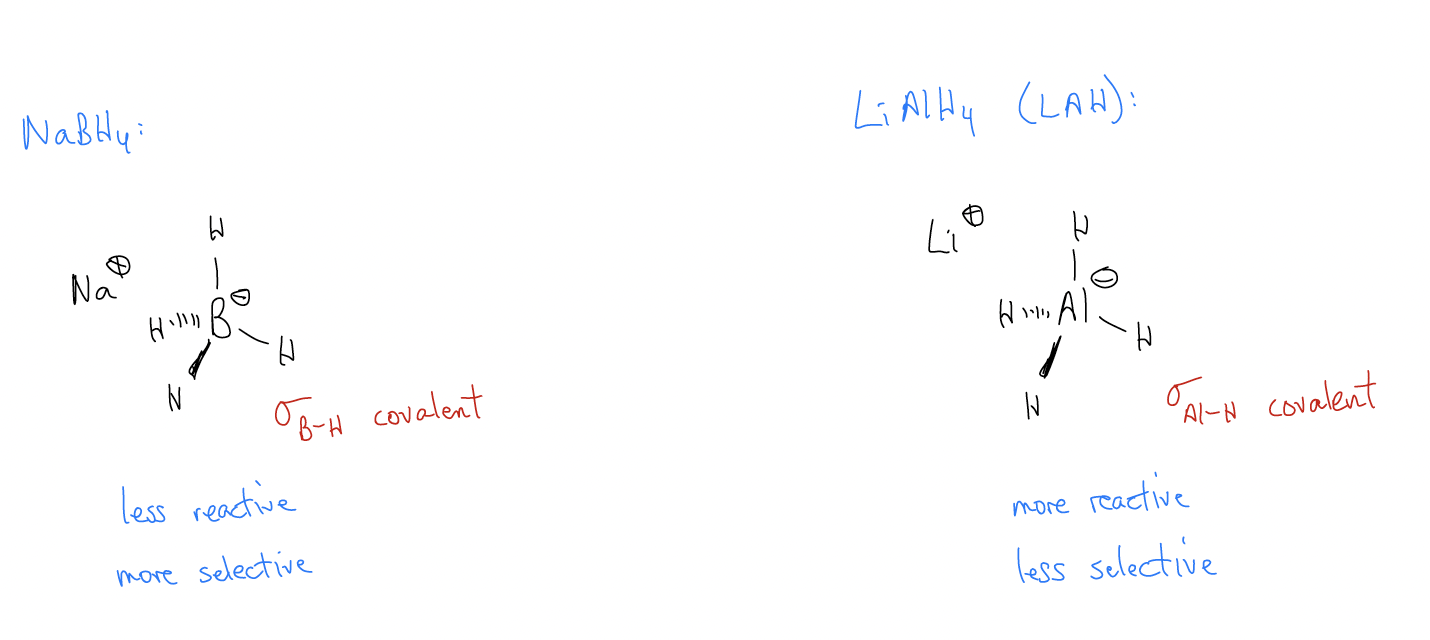
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Let’s first take a look at the reduction of ketones/aldehydes to produce 1°/2° alcohols. This can be performed by using a hydride equivalent. A hydride is a source of H–, but reagents like NaH or KH are not suitable to reduce ketones/aldehydes. The reason for this is that H– is too basic, the result of being a very hard, small point charge. It prefers to act like a base and deprotonate something than react like a nucleophile. The NaH bond is too ionic in character. Instead, we use hydride equivalents like sodium borohydride (NaBH4) or lithium aluminum hydride (LiAlH4 or LAH), which have \(σ\)B-H and \(σ\)Al-H bonds that are highly covalent and react by donating the hydrogen atom as a hydride equivalent, or “H–.” These reagents act as nucleophiles and deliver hydride to a ketone/aldehyde according to the same mechanism as above, generating a tetrahedral intermediate.
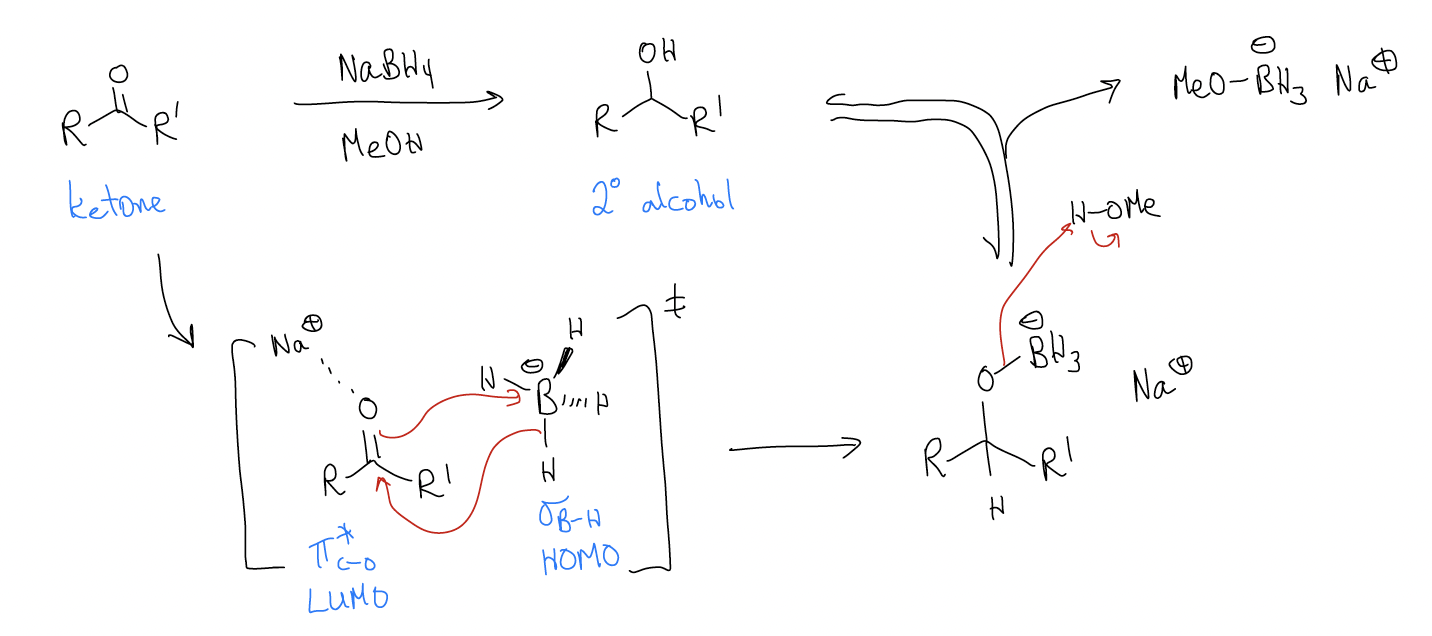
Let’s take a look at the mechanism of these reactions:
NaBH4 reduction:
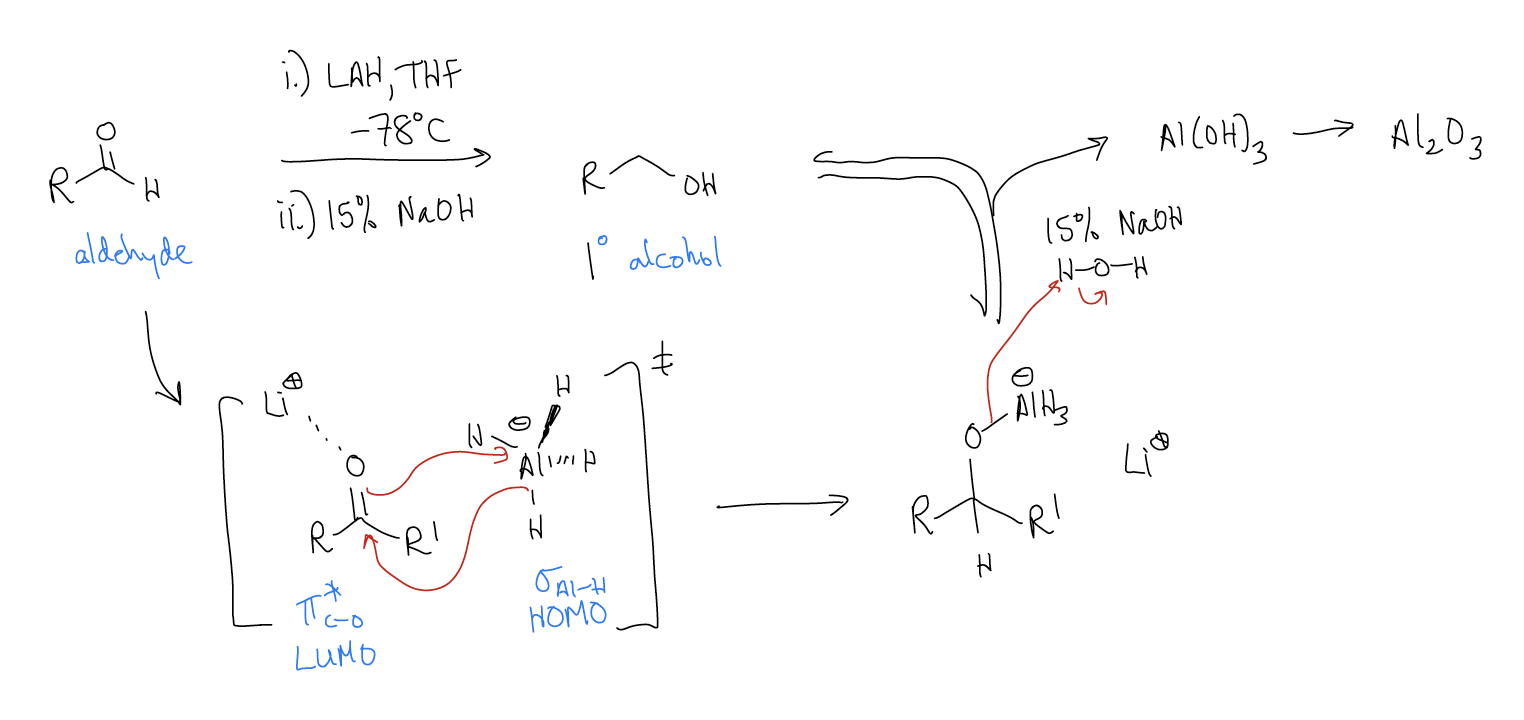
LiAlH4 reduction:
NaBH4 is less reactive but more selective than LAH. By analogy, LAH is more reactive but less selective. This makes for some interesting chemoselectivities. While both reagents are capable of reducing ketones/aldehydes to alcohols, only LAH is capable of reducing carboxylic acids to the corresponding alcohol (more on this mechanism later).
But let’s take a moment to think about the transition state for this transformation. Consider the reduction of 4-tert-butylcyclohexanone with LAH to give two diastereomers: A and B. These reduction products have different energies, and their formation is driven by the attack of the \(σ\)Al-H bond (HOMO) onto either the top or bottom face of the carbonyl \(π^{*}\)C-O (LUMO). These different faces make the transition state energies diastereomeric (and thus, different in energy).
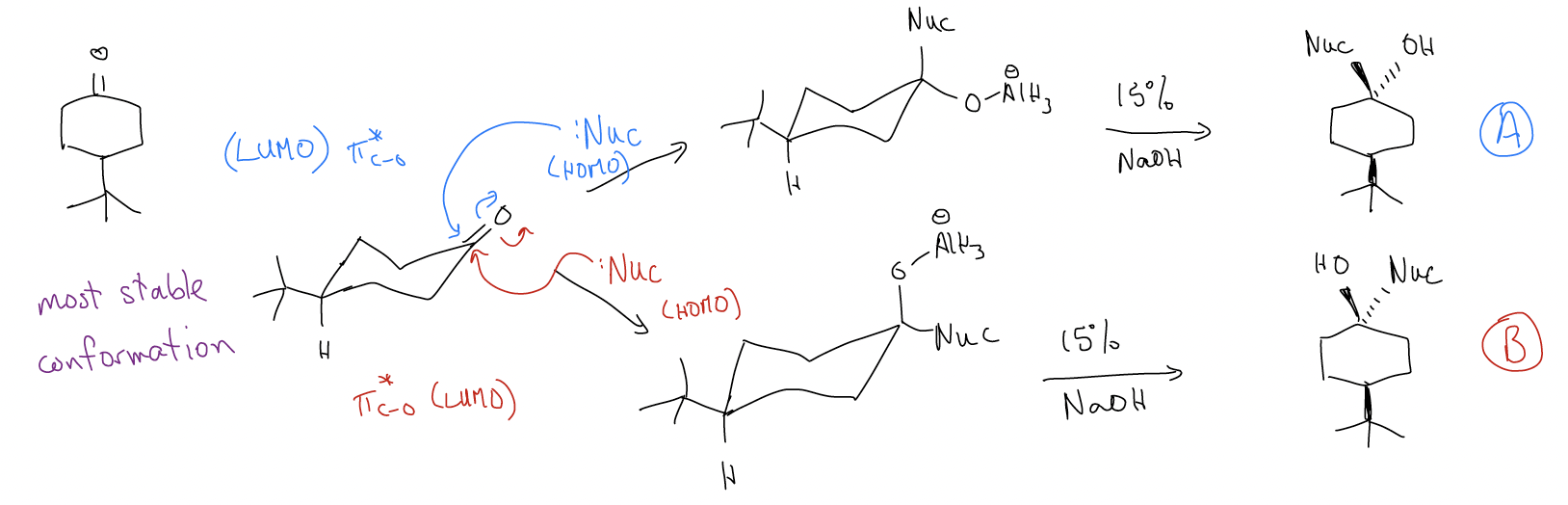
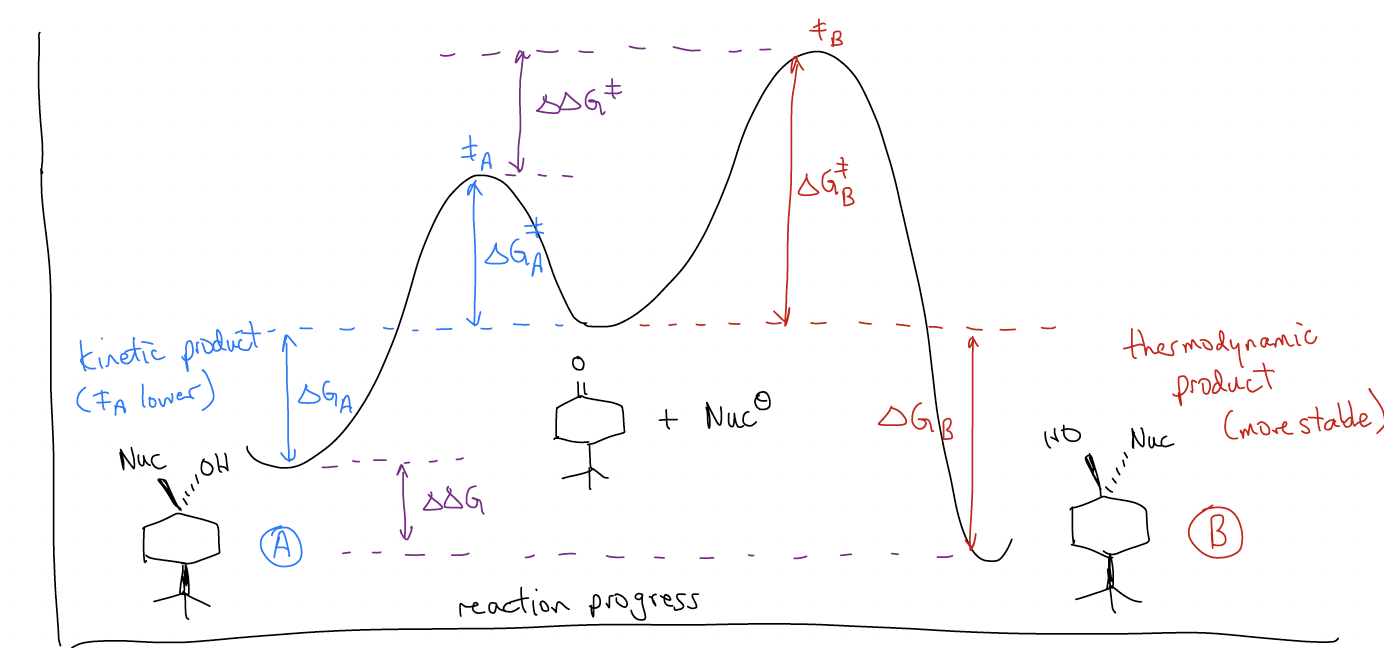
In the LAH reduction of 4-tert-butylcyclohexanone, the product distribution is 30:70 in favor of A. This does not change, even when heating. This should indicate to us that this reaction is irreversible (otherwise we would get B since it is lower in energy). We say that since the energies of the products have no effect on the product distribution and the energy of the transition state is controlling the reaction, that the reaction is under kinetic control.
So, why is this reaction irreversible? Let’s take a look at the mechanism and see if each step is itself reversible. In order for the reaction to be under thermodynamic control, the nucleophilic addition step must be reversible. This would mean “H–” must be a leaving group. We know that this can’t happen (pKaH = 32), so this step is under kinetic control. In the second step, protonation of the alkoxide gives the product alcohol. Protonations are reversible since protons can tunnel, so this step is under thermodynamic control (normally the protonated alcohol is more stable). Overall, the reaction is under kinetic control since the first step is irreversible.
Besides hydride equivalents, there are other nucleophiles that can add to carbonyls irreversibly. Grignard reagents and organolithium reagents also add to the \(π^{*}\) orbital (LUMO) efficiently under kinetic control. These reactions are irreversible in the first step because the reverse reaction would require a carbon-based anion (pKaH > 50) to be a good leaving group. Nucleophilic additions can only be under thermodynamic control if the incoming nucleophile is also a good leaving group (this time, the pKa must be < 15). The difference between reduction of ketones/aldehydes (“H–” addition) and addition of Grignards/organolithiums is that the resulting product is a 2°/3° alcohol after quenching with a proton source (normally NH4Cl).
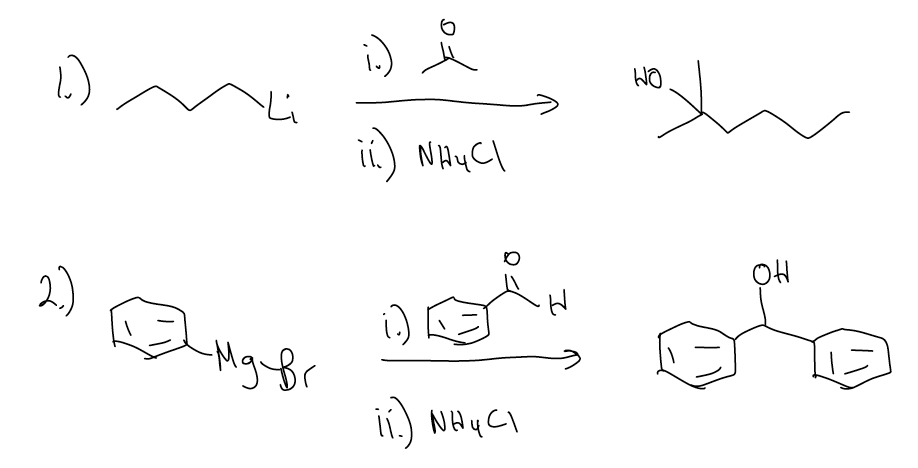
How does it work? It’s the same mechanism – nucleophilic addition to the carbonyl.
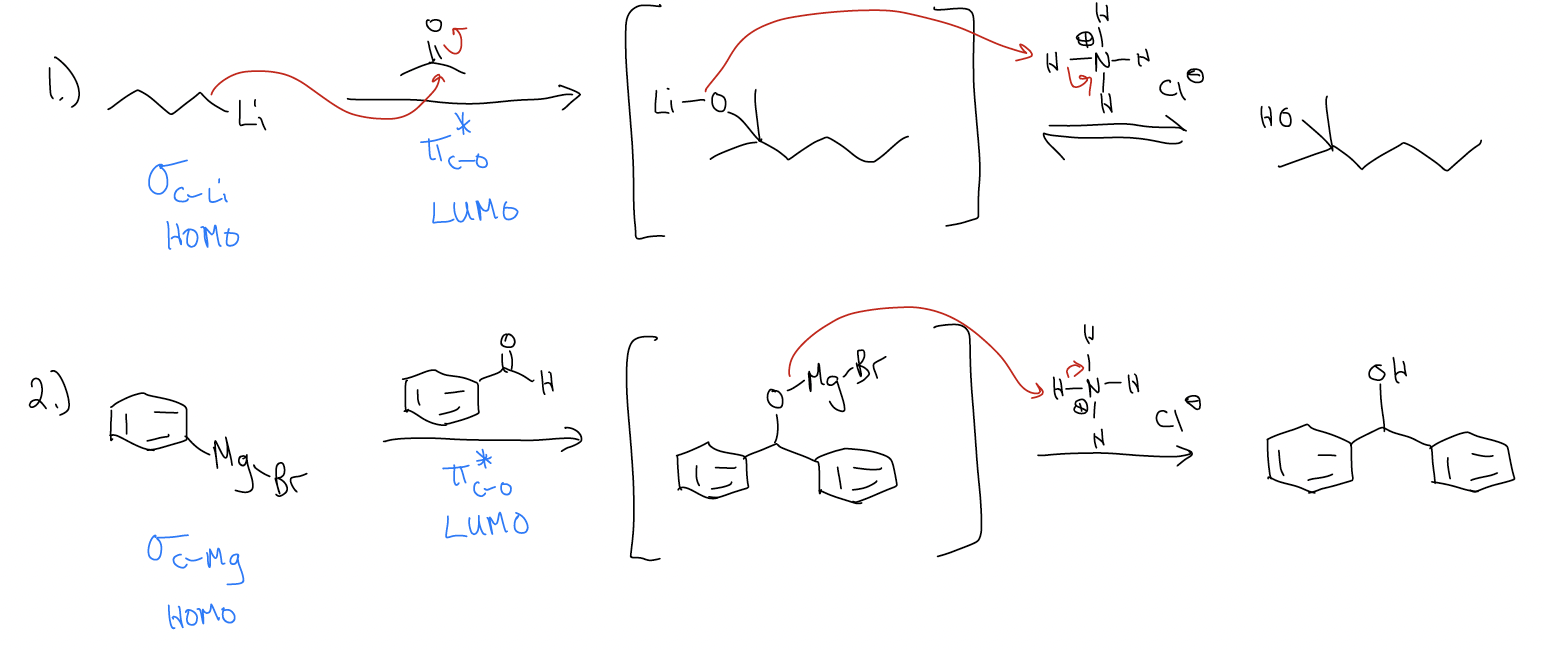
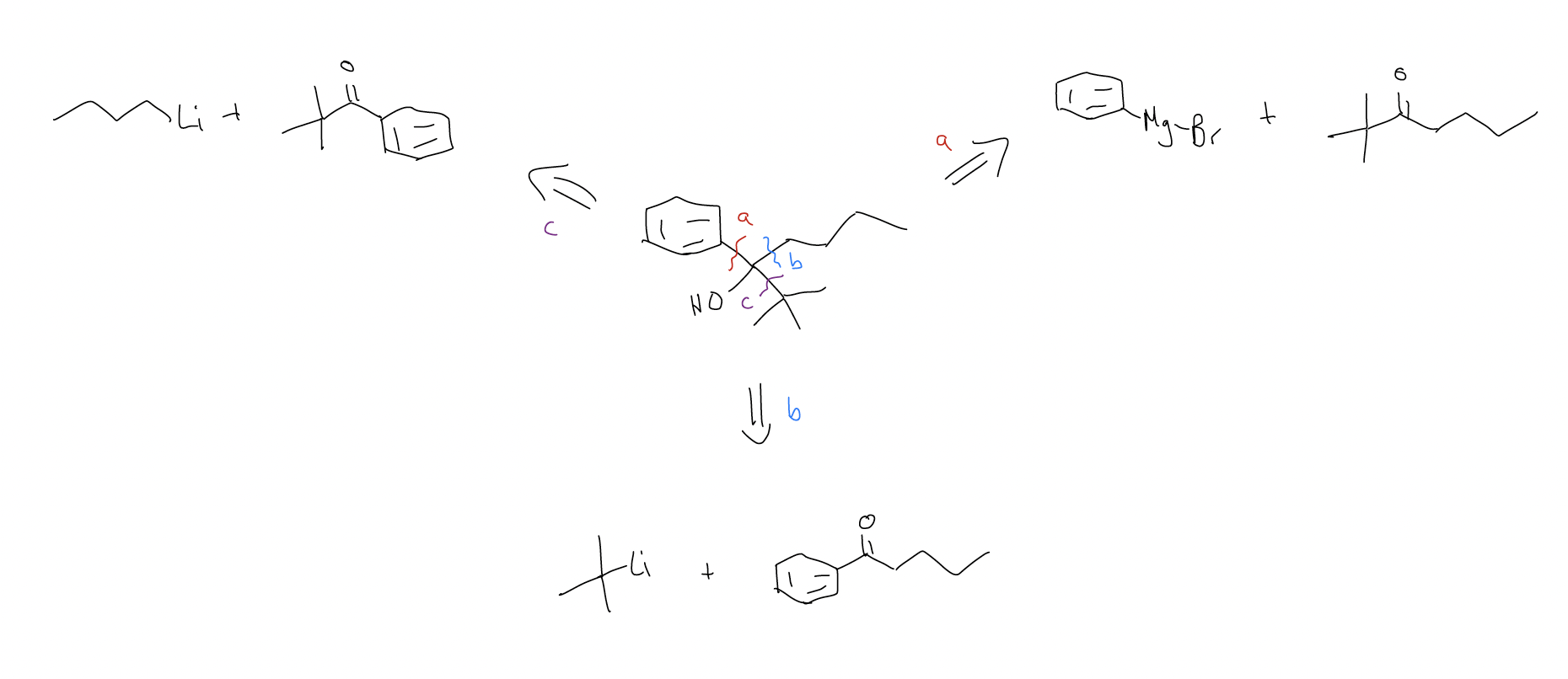
Tertiary alcohols (or secondary alcohols) can be made several different ways. Can you devise three different ways of making the following tertiary alcohol?