10.2: Concerted Cycloadditions
- Page ID
- 321638
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)A concerted cycloaddition is a reaction in which electron flow occurs in a circle or ring-like form, when bond-making and bond-breaking are simultaneous. The nature of the electrophile will dictate the size of the ring that forms and what functional group transformations will occur. For example, a [2+1] cycloaddition involves a 3-membered ring transition state where two atoms of the ring come from the alkene substrate (HOMO, nucleophile) and one atom of the ring comes from the electrophile. We will now go through each of these examples.
A. [2+1] cycloadditions
1. Halogenation
The bromination reaction involves formal addition of Br2 across the double bond. The mechanism is a [2+1] cycloaddition followed by an SN2 reaction.
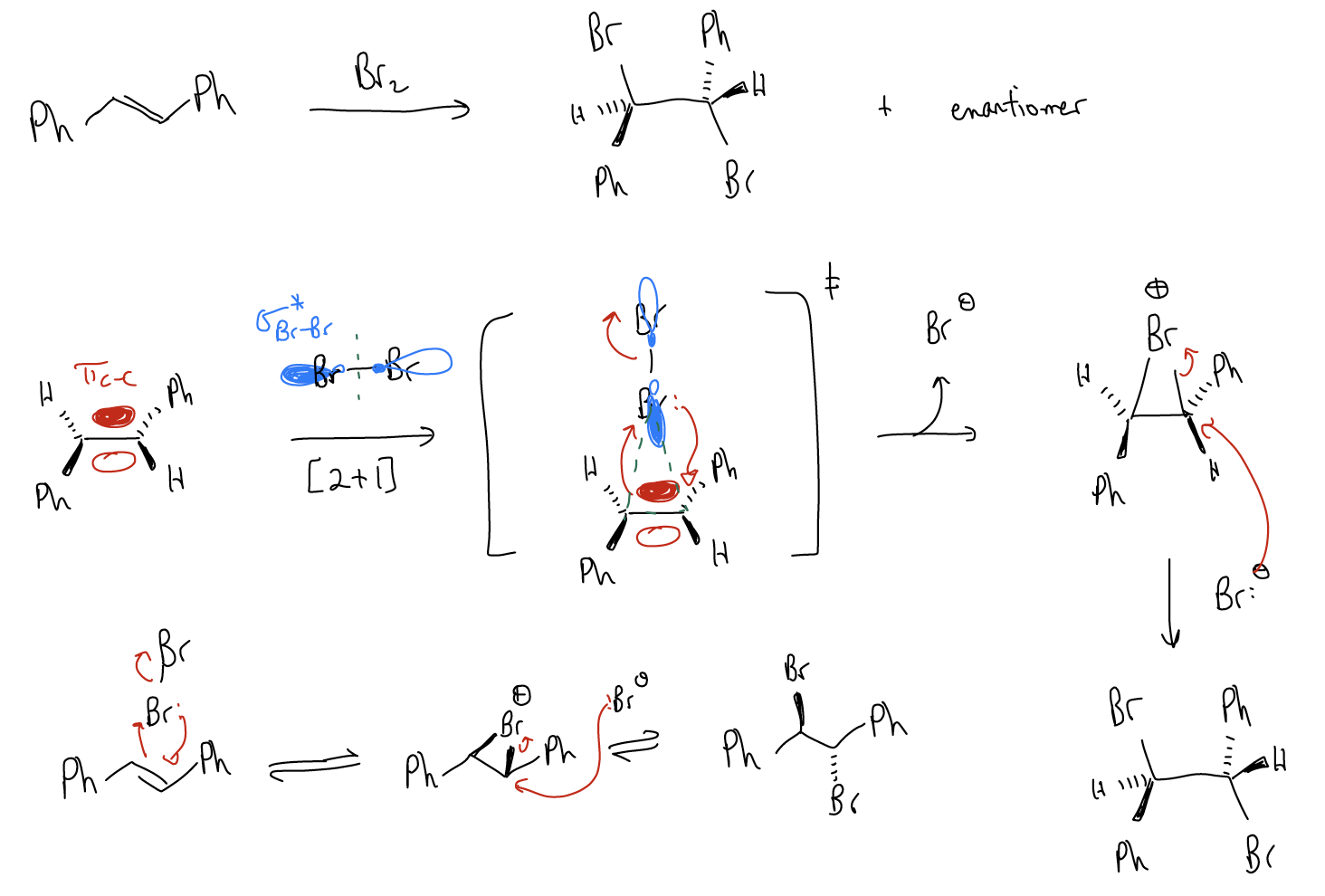
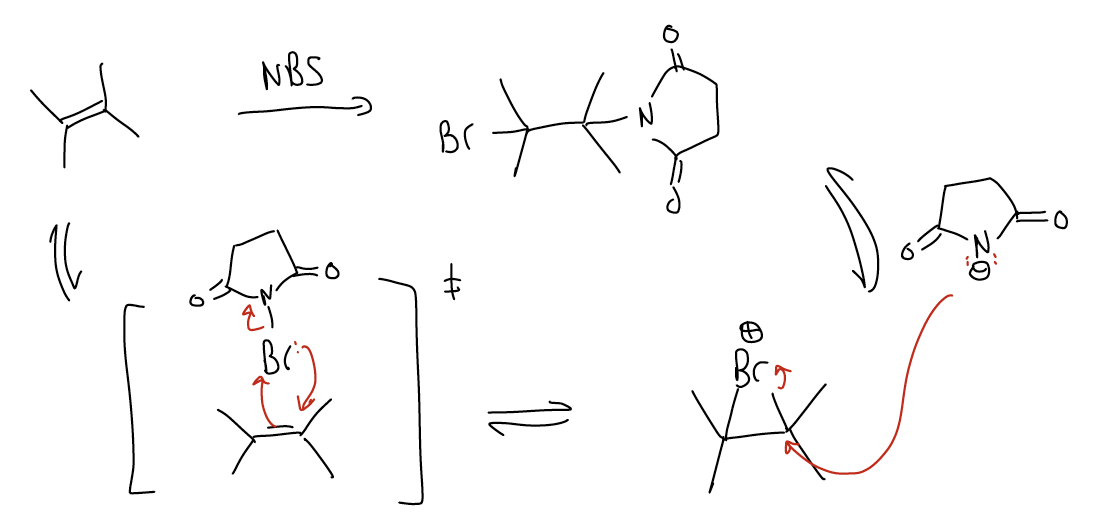
In the first step, the \(π_{C-C}\) (HOMO) acts as a nucleophile and populates the \(σ^{*}_{Br-Br}\), breaking the \(σ_{Br-Br}\) bond. At the same time, the lone pairs (nBr) will form a bond with the other carbon atom. We say that the bromine is now bridged, and the net result is a bromonium ion. There is equal bond formation between both carbon atoms. Notice that there is NEVER formation of a carbocation. The reaction is concerted and the addition of bromine is stereospecific. In the second step, bromide (Br–) will perform an SN2 reaction by attacking the \(σ^{*}_{C-Br}\). The net result (also stereospecific) is that there is anti addition across the double bond to give a trans dibromide. The fact that we get one product tells us that it is a concerted reaction. If it was not a concerted process and the bridged bromonium ion opened up, then we would get multiple products (from rotation about the s bond), such as the syn dibromide. Bromination of alkenes is a good method of testing a sample for the presence of double bonds because the brown color of Br2 will disappear upon reacting with alkenes to give a dibromide. Another method of bromination is with N-bromosuccinimide (NBS). There are other electrophiles that will perform similar mechanisms but give different functional groups: Cl2, NCS, I2, and NIS. In the case of chlorination, there is some stereochemical leakage (some competing halonium ion ring opening), and in the case of iodination, it can only be done intramolecularly.
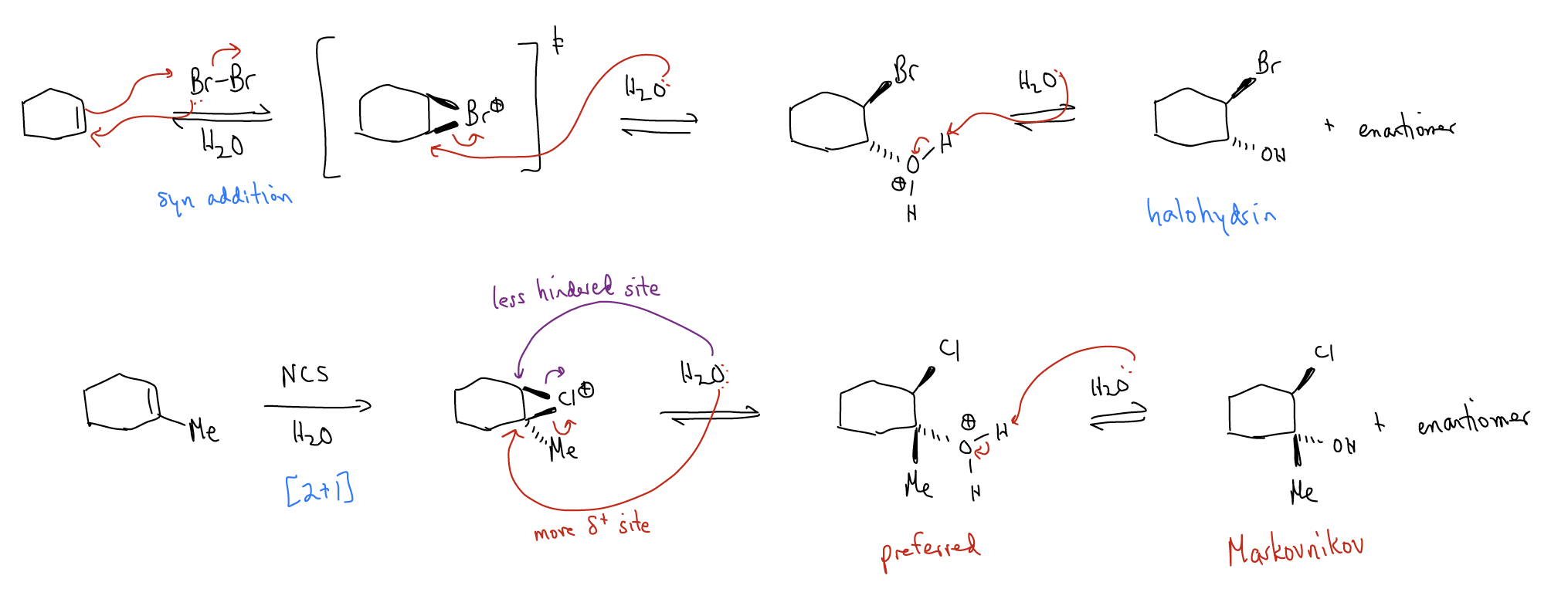
2. Halohydrin formation
If a halogenation reaction is performed in H2O (or some other nucleophilic solvent), a halohydrin can form. The stereochemical outcome is critical. Since the halonium ion must be cis, and SN2 reaction give the trans product stereospecifically. The regiochemical outcome could be described in two ways: attack of the halonium ion at the less sterically hindered site, or attack at the site that is more \(δ^{+}\). All halohydrins form with Markovnikov regioselectivity because H2O will attack at the site that is more \(δ^{+}\).
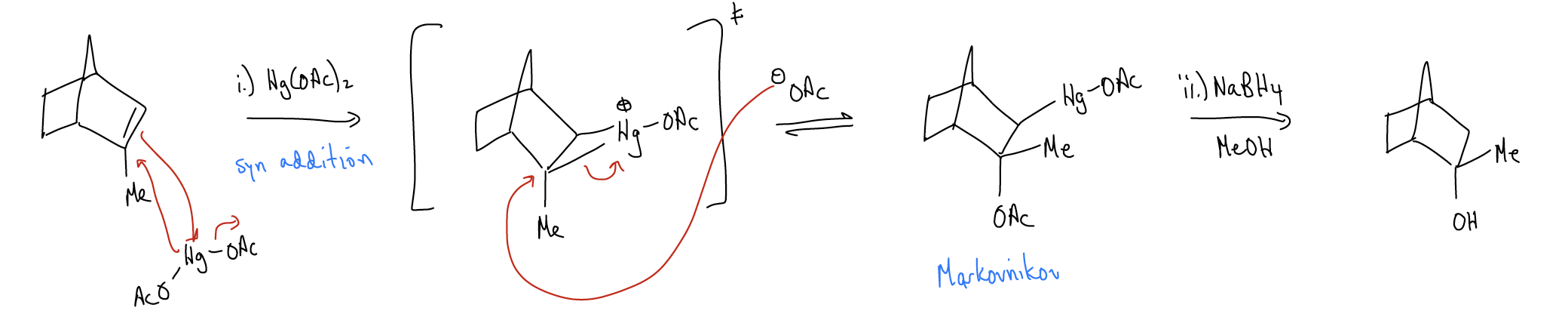
3. Oxymercuration
The reaction between an alkene and a mercury(II) salt is known as an oxymercuration. The mercurium ion also bridges, and is opened up by the acetate anion. Removal of the mercury can be accomplished by treating the reaction in a workup step with NaBH4 in methanol. This will replace the \(σ_{C-Hg}\) with a \(σ_{C-H}\) bond.
4. Epoxidation
The reaction between an alkene and a peroxyacid is known as an epoxidation. The product of the reaction is an epoxide, a three-membered ring containing oxygen. The reaction is stereospecific and takes advantage of the low-lying \(σ^{*}_{O-O}\) bond. Just like in halogenation, a bridged system results, but this time it is not charged. The most common reagent for this is meta-chloroperoxybenzoic acid (mCPBA).
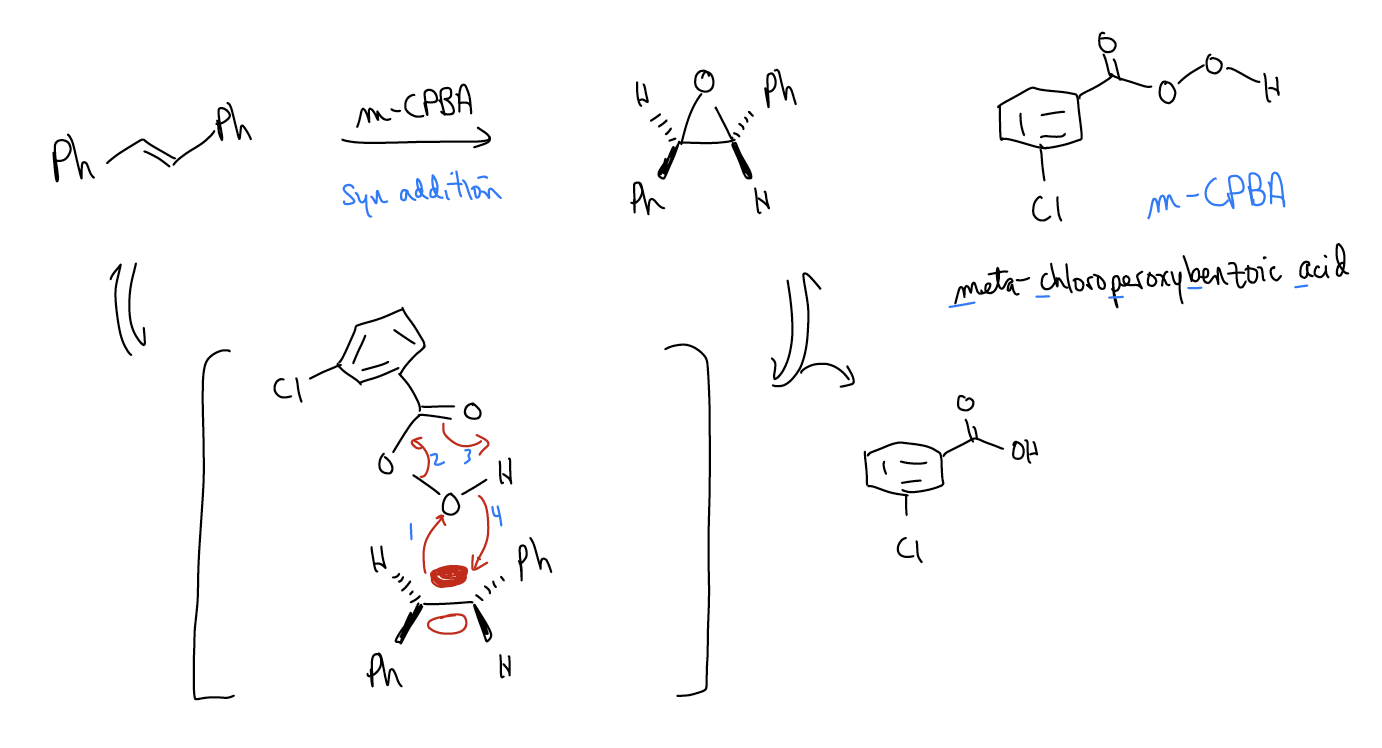
Epoxides are quite useful synthetic intermediates, since they readily open via SN2.
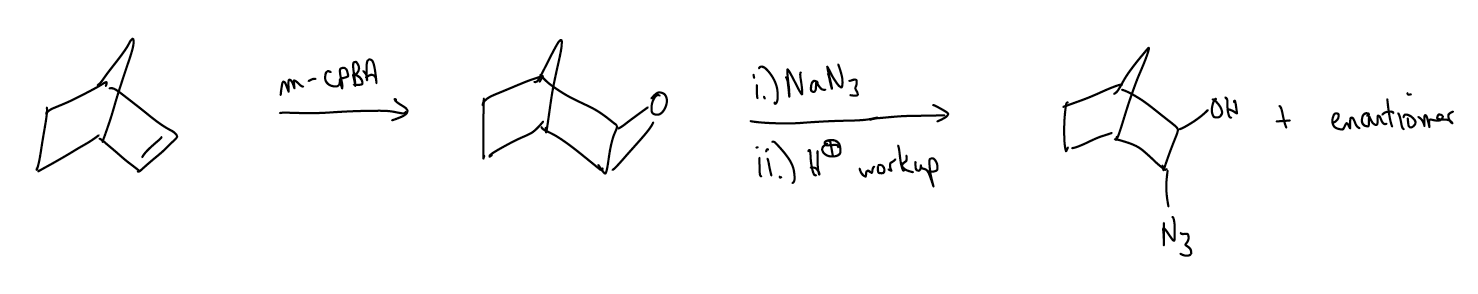
5. Carbenes and Carbenoids
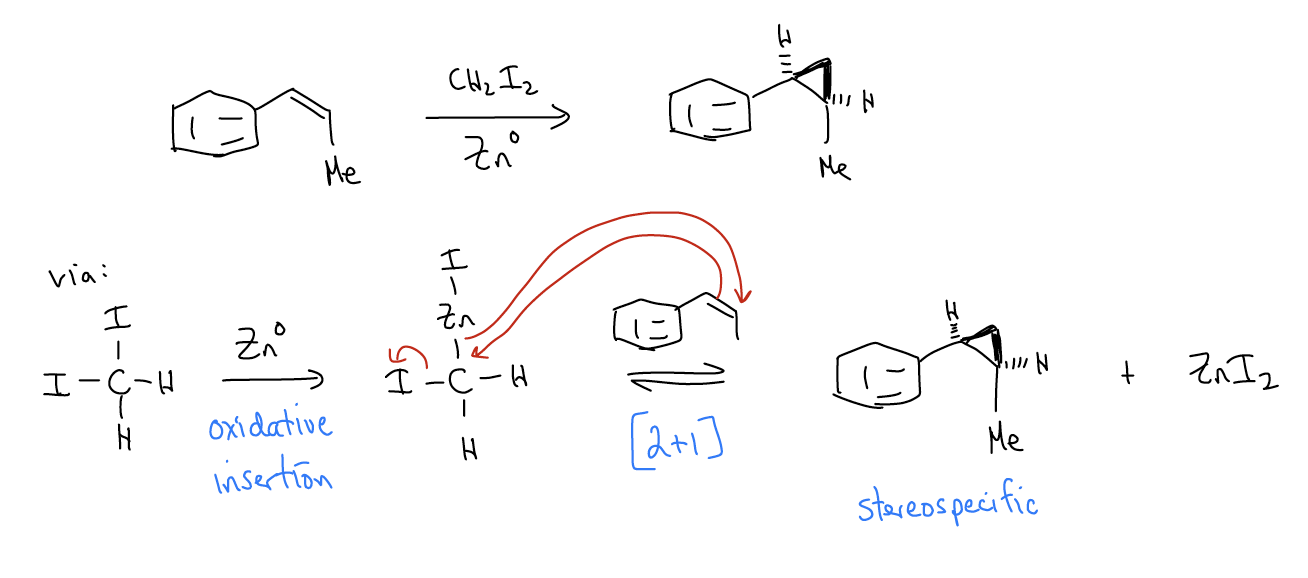
Carbenes are another reactive intermediate in organic chemistry (in addition to anions, cations, and radicals of carbon). They are divalent carbon species that are overall neutral, meaning they must have both a lone pair and an empty atomic p orbital. These intermediates participate in [2+1] cycloaddition reactions with alkenes, much like m-CPBA, to give cyclopropanes. One example is the carbene formed when using diazo compounds, as shown below:
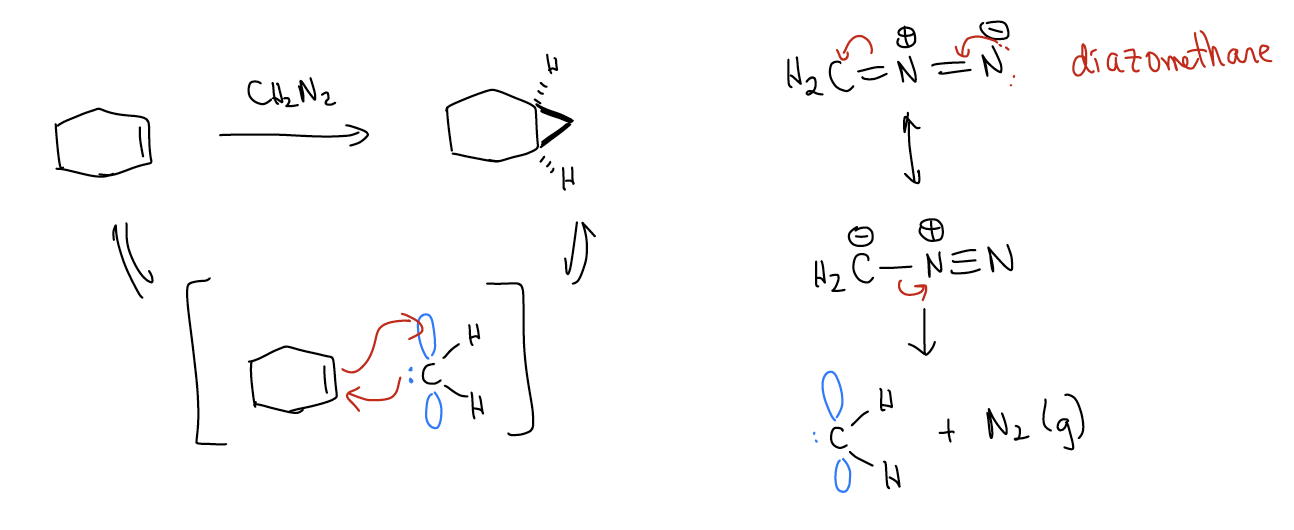
While there are some free carbenes, most reagents that have carbene-like reactivity are bound to a metal and are called carbenoids. One example is the Simmons-Smith reaction, which involves iodomethylzinc iodide.
B. [2+2] cycloadditions
1. Hydroboration/oxidation
The mechanism of the two-step hydroboration/oxidation involves a 4-membered ring transition state in the first step. The addition of BH3 across the double bond occurs so that the less highly substituted carbon is bound to boron. Because of this, when the \(σ_{C-B}\) bond is replaced with a \(σ_{C-O}\) bond in the product, we get the alcohol substitution on the less highly substituted carbon. Thus, this reaction has anti-Markovnikov regioselectivity (which is the exact opposite of a hydration reaction). The mechanism is found below:
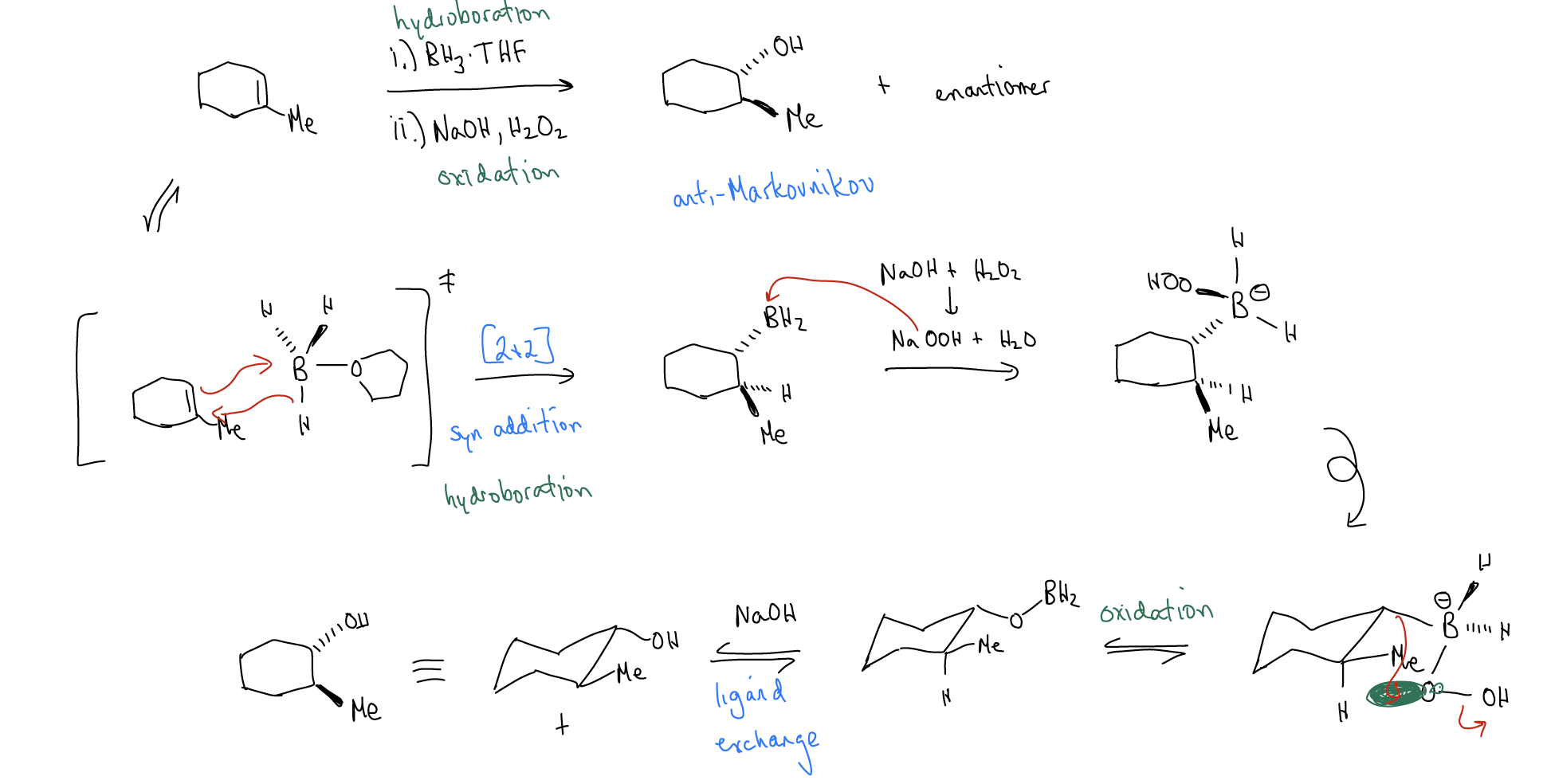
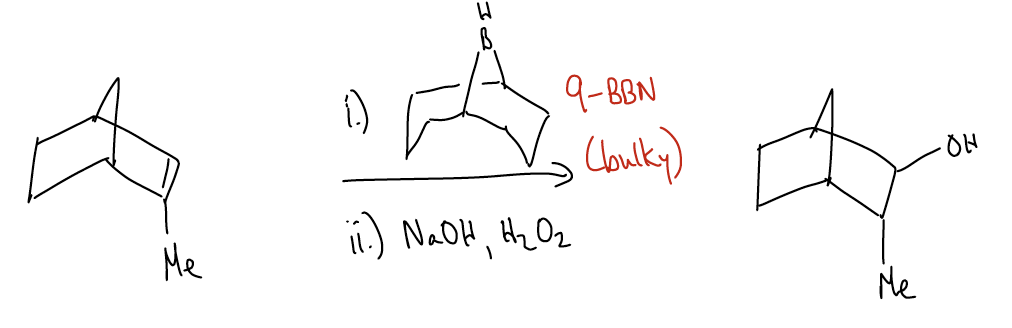
Why does the reaction occur with anti-Markovnikov regiochemistry? Consider the reacting orbitals: \(π_{C-C}\) (HOMO) and atomic pB (LUMO). The carbon with the greatest electron density (coefficient in the wavefunction) is the less highly substituted carbon due to \(σ\)-donation. The most electron deficient orbital is the atomic p on boron, so that pairing gives you the best overlap. Steric factors also contribute to this regiochemistry, especially is the boron is attached to big groups, as in the example below:
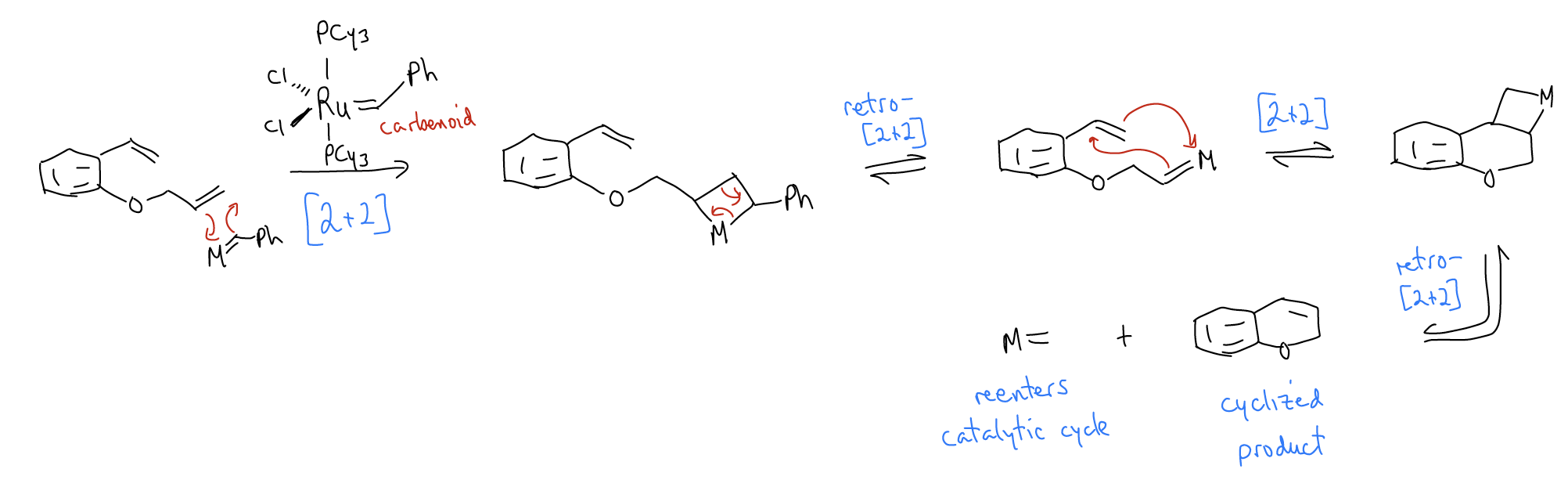
2. Olefin metathesis
Another example of a [2+2] cycloaddition is the olefin metathesis reaction, which uses a complex of Ru, W, or Mo. These reactions are especially useful for forming larger rings from two alkenes. This reaction also makes use of a carbenoid intermediate.
C. [3+2] cycloadditions
1. Ozonolysis
In an ozonolysis reaction, an alkene is converted into two different carbonyls (aldehydes or ketones, depending on the substitution pattern of the alkene). A 5-membered ring transition state brings together the alkene and ozone at low temperature to form an ozonide. This then breaks apart and rearranges to form a second ozonide. Workup with either dimethylsulfide (or LiAlH4, commonly known as LAH) results in formation of two different carbonyls and dimethylsulfoxide. This is a convenient way of making aldehydes/ketones.
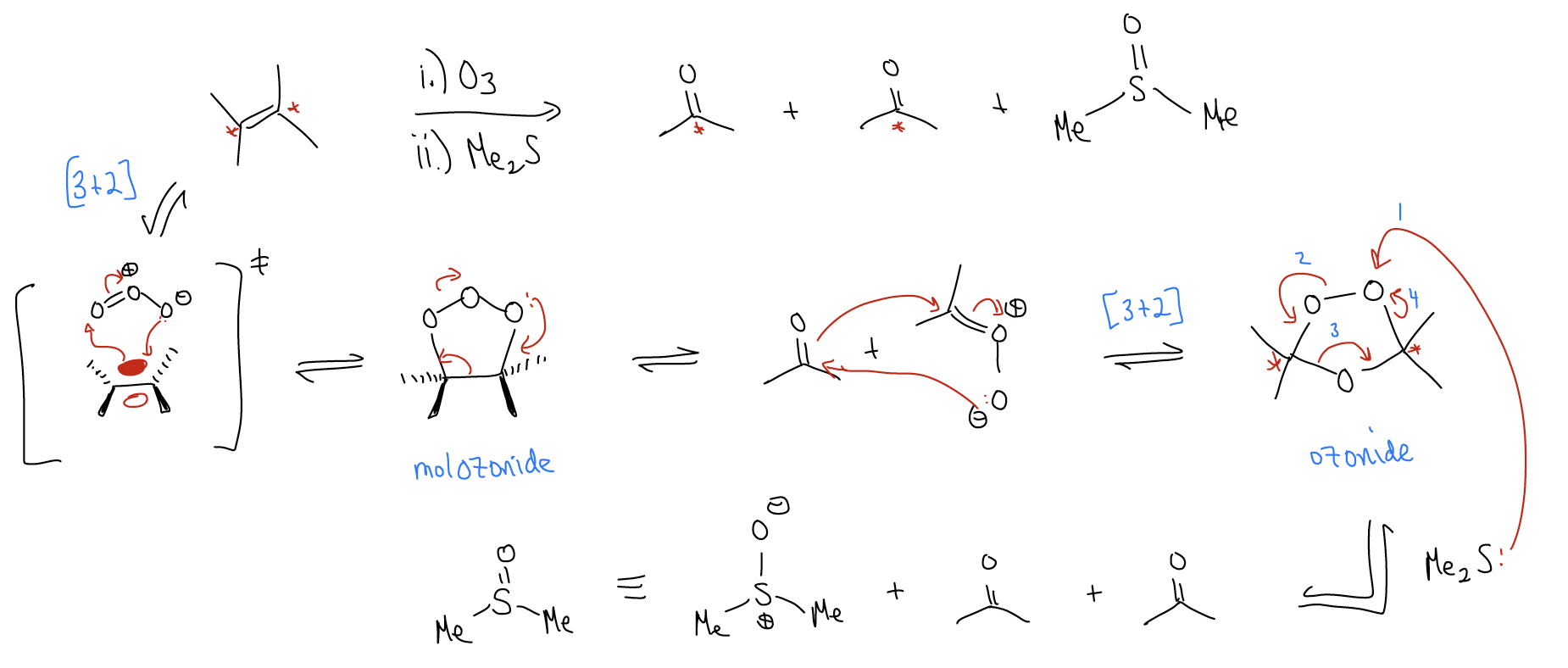
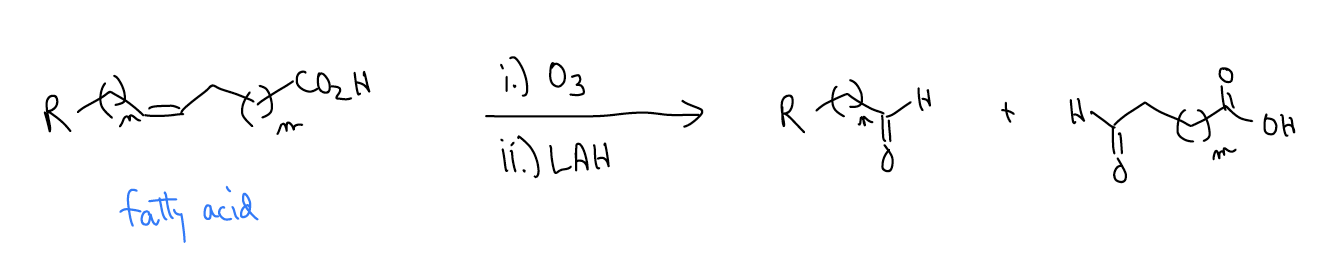
Ozonolysis is a convenient way of analyzing fatty acid content in food, since the lengths of the resulting carbonyls can be analyzed by mass spectrometry.
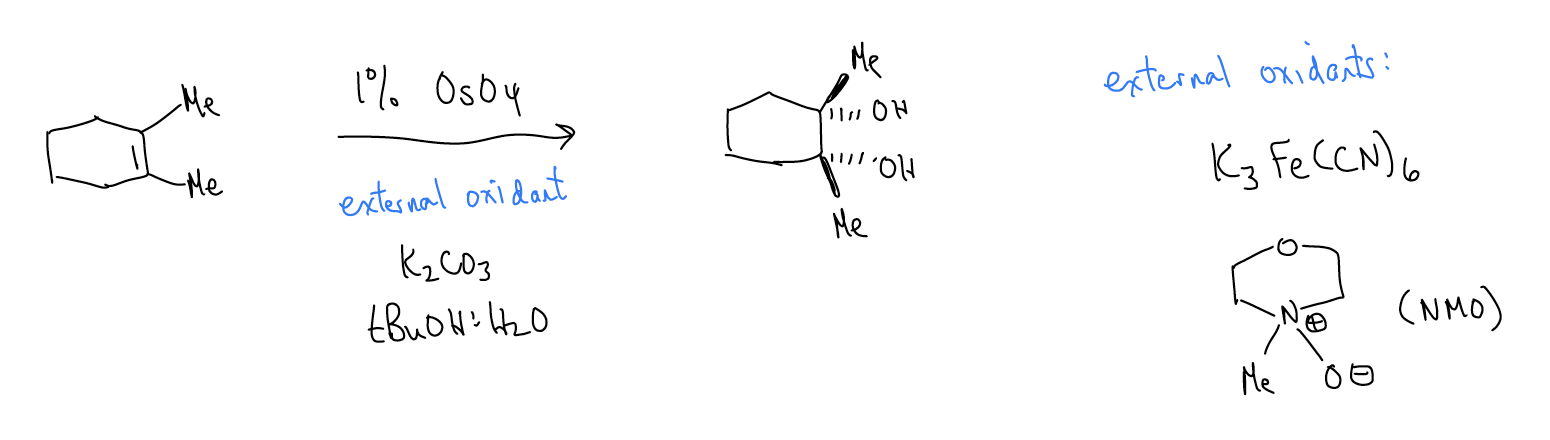
2. Dihydroxylation
In a dihydroxylation reaction, osmium tetroxide reacts with an alkene to form an osmate ester, which then hydrolyzes to give a syn diol. Because of the 5-membered ring transition state, the delivery of the two alcohol functional groups occurs from the same face of the alkene.
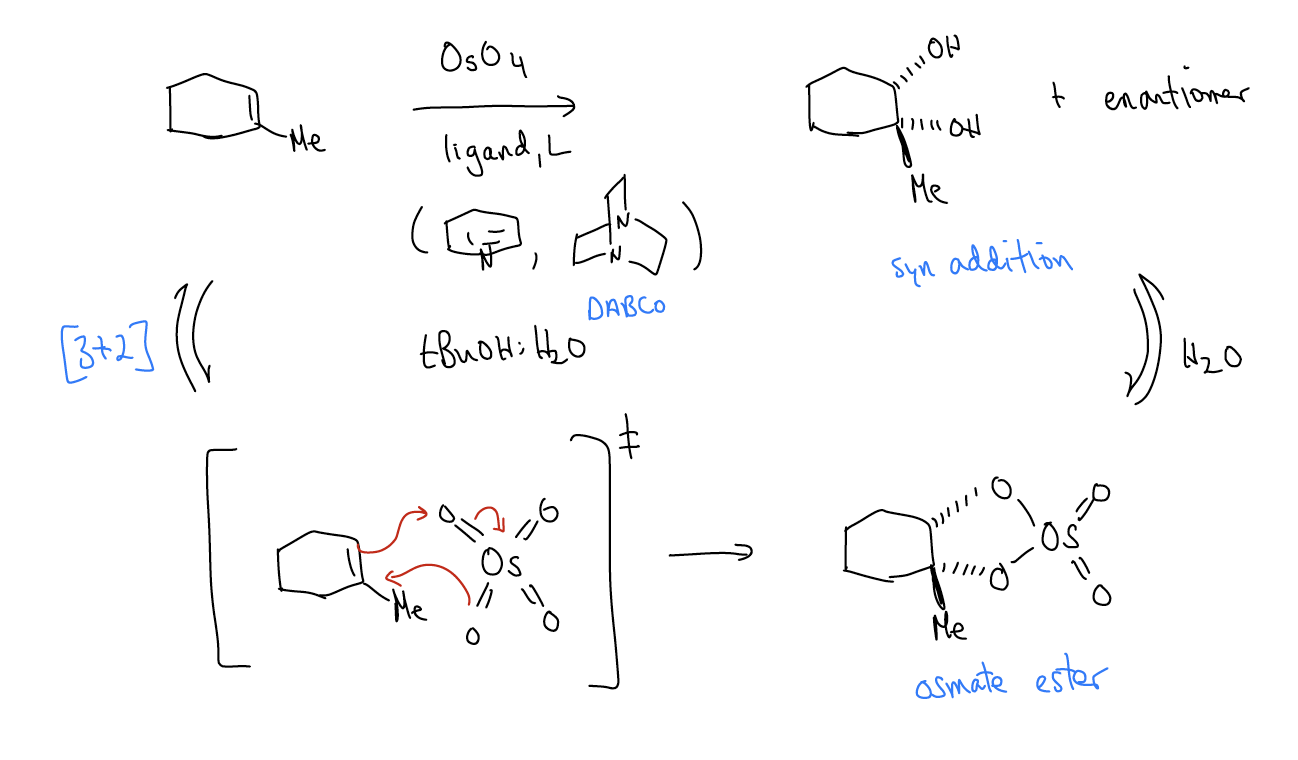
While this reaction can be performed stoichiometrically, it can also be performed catalytically if using a stoichiometric oxidant like K3Fe(CN)6 or N-methylmorpholine N-oxide (NMO).