Chapter 14.3: Solving Equilibrium Problems
- Page ID
- 42120
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\) |
Prince George's Community College |
 |
| Unit I: Atoms Unit II: Molecules Unit III: States of Matter Unit IV: Reactions Unit V: Kinetics & Equilibrium Unit VI: Thermo & Electrochemistry Unit VII: Nuclear Chemistry |
||
Learning Objectives
- To solve quantitative problems involving chemical equilibriums
There are two fundamental kinds of equilibrium problems: (1) those in which we are given the concentrations of the reactants and the products at equilibrium (or, more often, information that allows us to calculate these concentrations), and we are asked to calculate the equilibrium constant for the reaction; and (2) those in which we are given the equilibrium constant and the initial concentrations of reactants, and we are asked to calculate the concentration of one or more substances at equilibrium. In this section, we describe methods for solving both kinds of problems.
Calculating an Equilibrium Constant from Equilibrium Concentrations
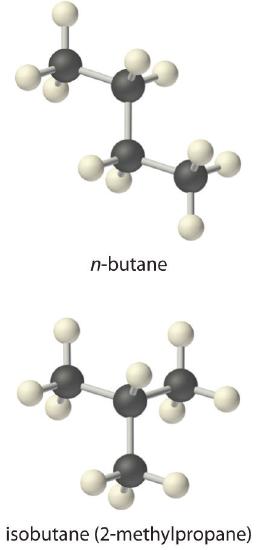 We saw in the exercise in Example 6 in Section 14.2 that the equilibrium constant for the decomposition of CaCO3(s) to CaO(s) and CO2(g) is K = [CO2]. At 800°C, the concentration of CO2 in equilibrium with solid CaCO3 and CaO is 2.5 × 10−3 M. Thus K at 800°C is 2.5 × 10−3. (Remember that equilibrium constants are unitless.)
We saw in the exercise in Example 6 in Section 14.2 that the equilibrium constant for the decomposition of CaCO3(s) to CaO(s) and CO2(g) is K = [CO2]. At 800°C, the concentration of CO2 in equilibrium with solid CaCO3 and CaO is 2.5 × 10−3 M. Thus K at 800°C is 2.5 × 10−3. (Remember that equilibrium constants are unitless.)
A more complex example of this type of problem is the conversion of n-butane, an additive used to increase the volatility of gasoline, to isobutane (2-methylpropane). This reaction can be written as follows:
\( n-butane \left ( g \right ) \rightleftharpoons isobutane \left ( g \right ) \tag{14.3.1} \)
and the equilibrium constant K = [isobutane]/[n-butane]. At equilibrium, a mixture of n-butane and isobutane at room temperature was found to contain 0.041 M isobutane and 0.016 M n-butane. Substituting these concentrations into the equilibrium constant expression,
\( K= \dfrac{isobutane}{n-butane}=\dfrac{0.041\;\cancel{M}}{0.016 \; \cancel{M}} = 2.6 \tag{14.3.2} \)
Thus the equilibrium constant for the reaction as written is 2.6.
Example 14.3.1
The reaction between gaseous sulfur dioxide and oxygen is a key step in the industrial synthesis of sulfuric acid:
\( 2SO_{2}\left ( g \right ) + O_{2}\left ( g \right ) \rightleftharpoons 2SO_{3}\left ( g \right ) \)
A mixture of SO2 and O2 was maintained at 800 K until the system reached equilibrium. The equilibrium mixture contained 5.0 × 10−2 M SO3, 3.5 × 10−3 M O2, and 3.0 × 10−3 M SO2. Calculate K and Kp at this temperature.
Given: balanced equilibrium equation and composition of equilibrium mixture
Asked for: equilibrium constant
Strategy:
Write the equilibrium constant expression for the reaction. Then substitute the appropriate equilibrium concentrations into this equation to obtain K.
Solution:
Substituting the appropriate equilibrium concentrations into the equilibrium constant expression,
\( K=\dfrac{\left [ SO_{3} \right ]^{2}}{\left [ SO_{2} \right ]^{2}\left [ O_{2} \right ]}=\dfrac{\left ( 5.0\times 10^{-2} \right )^{2}}{\left ( 3.0\times 10^{-3} \right )^{2}\left ( 3.5\times 10^{-3} \right )}=7.9\times 10^{4} \)
To solve for Kp, we use Equation 14.2.18, where Δn = 2 − 3 = −1:
\( K_{p}= K\left ( RT \right )^{\Delta n} \)
\( =7.9\times 10^{4}\left [ \left (0.082606\; L\cdot atm/mol\cdot \cancel{K} \right ) \left ( 800 \; \cancel{K} \right )\right ] \)
\( =1.2\times 10^{3}\)
Exercise
Hydrogen gas and iodine react to form hydrogen iodide via the reaction
\( H_{2}\left ( g \right ) + I_{2}\left ( g \right ) \rightleftharpoons 2HI\left ( g \right ) \)
A mixture of H2 and I2 was maintained at 740 K until the system reached equilibrium. The equilibrium mixture contained 1.37 × 10−2 M HI, 6.47 × 10−3 M H2, and 5.94 × 10−4 M I2. Calculate K and Kp for this reaction.
Answer: K = 48.8; Kp = 48.8
Chemists are not often given the concentrations of all the substances, and they are not likely to measure the equilibrium concentrations of all the relevant substances for a particular system. In such cases, we can obtain the equilibrium concentrations from the initial concentrations of the reactants and the balanced chemical equation for the reaction, as long as the equilibrium concentration of one of the substances is known. Example 9 shows one way to do this.
Example 14.3.2
A 1.00 mol sample of NOCl was placed in a 2.00 L reactor and heated to 227°C until the system reached equilibrium. The contents of the reactor were then analyzed and found to contain 0.056 mol of Cl2. Calculate K at this temperature. The equation for the decomposition of NOCl to NO and Cl2 is as follows:
\( 2NOCl \left ( g \right ) \rightleftharpoons 2NO\left ( g \right ) + Cl_{2}\left ( g \right ) \)
Given: balanced equilibrium equation, amount of reactant, volume, and amount of one product at equilibrium
Asked for: K
Strategy:
A Write the equilibrium constant expression for the reaction. Construct a table showing the initial concentrations, the changes in concentrations, and the final concentrations (as initial concentrations plus changes in concentrations).
B Calculate all possible initial concentrations from the data given and insert them in the table.
C Use the coefficients in the balanced chemical equation to obtain the changes in concentration of all other substances in the reaction. Insert those concentration changes in the table.
D Obtain the final concentrations by summing the columns. Calculate the equilibrium constant for the reaction.
Solution:
A The first step in any such problem is to balance the chemical equation for the reaction (if it is not already balanced) and use it to derive the equilibrium constant expression. In this case, the equation is already balanced, and the equilibrium constant expression is as follows:
\( K=\dfrac{\left [ NO_{2} \right ]^{2}\left [ Cl_{2} \right ]}{\left [ NOCl \right ]^{2}} \)
To obtain the concentrations of NOCl, NO, and Cl2 at equilibrium, we construct a table showing what is known and what needs to be calculated. We begin by writing the balanced chemical equation at the top of the table, followed by three lines corresponding to the initial concentrations, the changes in concentrations required to get from the initial to the final state, and the final concentrations.
| 2NOCl(g) ⇌ 2NO(g) + Cl2 | |||
|---|---|---|---|
| [NOCl] | [NO] | [Cl2] | |
| initial | |||
| change | |||
| final | |||
B Initially, the system contains 1.00 mol of NOCl in a 2.00 L container. Thus [NOCl]i = 1.00 mol/2.00 L = 0.500 M. The initial concentrations of NO and Cl2 are 0 M because initially no products are present. Moreover, we are told that at equilibrium the system contains 0.056 mol of Cl2 in a 2.00 L container, so [Cl2]f = 0.056 mol/2.00 L = 0.028 M. We insert these values into the following table:
| 2NOCl(g) ⇌ 2NO(g) + Cl2 | |||
|---|---|---|---|
| [NOCl] | [NO] | [Cl2] | |
| initial | 0.500 | 0 | 0 |
| change | |||
| final | 0.028 | ||
C We use the stoichiometric relationships given in the balanced chemical equation to find the change in the concentration of Cl2, the substance for which initial and final concentrations are known:
Δ[Cl2] = [0.028 M (final) − 0.00 M (initial)] = +0.028 M
According to the coefficients in the balanced chemical equation, 2 mol of NO are produced for every 1 mol of Cl2, so the change in the NO concentration is as follows:
\( \Delta \left [ NO \right ] = \left ( \dfrac{0.028 \; \cancel{mol\;Cl_{2}}}{L} \right )\left ( \dfrac{2\; mol\; NO}{1\;\cancel{mol\;Cl_{2}}} \right )=0.056\; M \)
Similarly, 2 mol of NOCl are consumed for every 1 mol of Cl2 produced, so the change in the NOCl concentration is as follows:
\( \Delta \left [ NOCl \right ] = \left ( \dfrac{0.028 \; \cancel{mol\;Cl_{2}}}{L} \right )\left ( \dfrac{-2\; mol\; NO}{1\;\cancel{mol\;Cl_{2}}} \right )=-0.056\; M \)
We insert these values into our table:
| 2NOCl(g) ⇌ 2NO(g) + Cl2 | |||
|---|---|---|---|
| [NOCl] | [NO] | [Cl2] | |
| initial | 0.500 | 0 | 0 |
| change | −0.056 | +0.056 | +0.028 |
| final | 0.028 | ||
D We sum the numbers in the [NOCl] and [NO] columns to obtain the final concentrations of NO and NOCl:
[NO]f = 0.000 M + 0.056 M = 0.056 M [NOCl]f = 0.500 M + (−0.056 M) = 0.444 M
We can now complete the table:
| 2NOCl(g) ⇌ 2NO(g) + Cl2 | |||
|---|---|---|---|
| [NOCl] | [NO] | [Cl2] | |
| initial | 0.500 | 0 | 0 |
| change | −0.056 | +0.056 | +0.028 |
| final | 0.444 | 0.056 | 0.028 |
We can now calculate the equilibrium constant for the reaction:
\( K=\dfrac{\left [ NO_{2} \right ]^{2}\left [ Cl_{2} \right ]}{\left [ NOCl \right ]^{2}}=\dfrac{\left ( 0.056 \right )^{2}\left ( 0.028 \right )}{0.444}^{2}=4.5\times 10^{-4} \)
Exercise
The German chemist Fritz Haber (1868–1934; Nobel Prize in Chemistry 1918) was able to synthesize ammonia (NH3) by reacting 0.1248 M H2 and 0.0416 M N2 at about 500°C. At equilibrium, the mixture contained 0.00272 M NH3. What is K for the reaction N2 + 3 H2 ⇌ 2NH3at this temperature? What is Kp?
Answer: K = 0.105; Kp = 2.61 × 10−5
The original laboratory apparatus designed by Fritz Haber and Robert Le Rossignol in 1908 for synthesizing ammonia from its elements. A metal catalyst bed, where ammonia was produced, is in the large cylinder at the left. The Haber-Bosch process used for the industrial production of ammonia uses essentially the same process and components but on a much larger scale. Unfortunately, Haber’s process enabled Germany to prolong World War I when German supplies of nitrogen compounds, which were used for explosives, had been exhausted in 1914.
Calculating Equilibrium Concentrations from the Equilibrium Constant
To describe how to calculate equilibrium concentrations from an equilibrium constant, we first consider a system that contains only a single product and a single reactant, the conversion of n-butane to isobutane (Equation 14.3.3), for which K = 2.6 at 25°C. If we begin with a 1.00 M sample of n-butane, we can determine the concentration of n-butane and isobutane at equilibrium by constructing a table showing what is known and what needs to be calculated, just as we did in Example 14.3.2.
\[ n-butane\left (g \right )\rightleftharpoons isobutane\left ( g \right ) \tag{14.3.3} \]
| nbutane(g) ⇌isobutane(g) | ||
|---|---|---|
| [n-Butane] | [Isobutane] | |
| initial | ||
| change | ||
| final | ||
The initial concentrations of the reactant and product are both known: [n-butane]i = 1.00 M and [isobutane]i = 0 M. We need to calculate the equilibrium concentrations of both n-butane and isobutane. Because it is generally difficult to calculate final concentrations directly, we focus on the change in the concentrations of the substances between the initial and the final (equilibrium) conditions. If, for example, we define the change in the concentration of isobutane (Δ[isobutane]) as +x, then the change in the concentration of n-butane is Δ[n-butane] = −x. This is because the balanced chemical equation for the reaction tells us that 1 mol of n-butane is consumed for every 1 mol of isobutane produced. We can then express the final concentrations in terms of the initial concentrations and the changes they have undergone.
| nbutane(g) ⇌isobutane(g) | ||
|---|---|---|
| [n-Butane] | [Isobutane] | |
| initial | 1.00 | 0 |
| change | −x | +x |
| final | (1.00 − x) | (0 + x) = x |
Substituting the expressions for the final concentrations of n-butane and isobutane from the table into the equilibrium equation,
\( K=\dfrac{\left [ isobutane \right]}{\left [ n-butane \right ]}=\dfrac{x}{1.00-x}=2.6 \)
Rearranging and solving for x,
\( x = 2.6\left ( 1.00-x \right )=2.6-2.6x \)
\( x + 2.6x =2.6 \)
\( x = 0.72 \)
We obtain the final concentrations by substituting this x value into the expressions for the final concentrations of n-butane and isobutane listed in the table:
[n-butane]f = (1.00 − x) M = (1.00 − 0.72) M = 0.28 M [isobutane]f = (0.00 + x) M = (0.00 + 0.72) M = 0.72 M
We can check the results by substituting them back into the equilibrium constant expression to see whether they give the same K that we used in the calculation:
\( K=\dfrac{\left [ isobutane \right]}{\left [ n-butane \right ]}=\dfrac{0.72 \; \cancel{M}}{0.28 \; \cancel{M}}=2.6 \)
This is the same K we were given, so we can be confident of our results.
Example 14.3.3 illustrates a common type of equilibrium problem that you are likely to encounter.
Example 14.3.3
The water–gas shift reaction is important in several chemical processes, such as the production of H2 for fuel cells. This reaction can be written as follows:
\( H_{2}\left ( g \right ) + CO_{2}\left ( g \right ) \rightleftharpoons H_{2}O\left ( g \right ) + CO\left ( g \right )\)
K = 0.106 at 700 K. If a mixture of gases that initially contains 0.0150 M H2 and 0.0150 M CO2 is allowed to equilibrate at 700 K, what are the final concentrations of all substances present?
Given: balanced equilibrium equation, K, and initial concentrations
Asked for: final concentrations
Strategy:
A Construct a table showing what is known and what needs to be calculated. Define x as the change in the concentration of one substance. Then use the reaction stoichiometry to express the changes in the concentrations of the other substances in terms of x. From the values in the table, calculate the final concentrations.
B Write the equilibrium equation for the reaction. Substitute appropriate values from the table to obtain x.
C Calculate the final concentrations of all species present. Check your answers by substituting these values into the equilibrium constant expression to obtain K.
Solution:
A The initial concentrations of the reactants are [H2]i = [CO2]i = 0.0150 M. Just as before, we will focus on the change in the concentrations of the various substances between the initial and final states. If we define the change in the concentration of H2O as x, then Δ[H2O] = +x. We can use the stoichiometry of the reaction to express the changes in the concentrations of the other substances in terms of x. For example, 1 mol of CO is produced for every 1 mol of H2O, so the change in the CO concentration can be expressed as Δ[CO] = +x. Similarly, for every 1 mol of H2O produced, 1 mol each of H2 and CO2 are consumed, so the change in the concentration of the reactants is Δ[H2] = Δ[CO2] = −x. We enter the values in the following table and calculate the final concentrations.
| H2(g) + CO2(g) ⇌ H2O(g) + CO(g) | ||||
|---|---|---|---|---|
| [H2] | [CO2] | [H2O] | [CO] | |
| initial | 0.0150 | 0.0150 | 0 | 0 |
| change | −x | −x | +x | +x |
| final | (0.0150 − x) | (0.0150 − x) | x | x |
B We can now use the equilibrium equation and the given K to solve for x:
\( K=\dfrac{\left [ H_{2}O] \right ] \left [ CO \right ]}{\left [ H_{2} \right ]\left [ CO_{2} \right ]}=\dfrac{\left (x \right )\left ( x \right ) }{\left ( 0.0150-x \right )\left ( 0.0150-x \right )}=\dfrac{x^{2}}{\left ( 0.0150-x \right )^{2}}=0.160 \)
We could solve this equation with the quadratic formula, but it is far easier to solve for x by recognizing that the left side of the equation is a perfect square; that is,
\[\dfrac{x^2}{(0.0150−x)^2}=\left(\dfrac{x}{0.0150−x}\right)^2=0.106\]
Taking the square root of the middle and right terms,
\[\dfrac{x^2}{(0.0150−x)^2} =(0.106)^{1/2}=0.326\]
\[x =(0.326)(0.0150)−0.326x\]
\[1.326x=0.00489\]
\[x =0.00369=3.69 \times 10^{−3}\]
C The final concentrations of all species in the reaction mixture are as follows:
- \([H_2]_f=[H_2]_i+Δ[H_2]=(0.0150−0.00369) \;M=0.0113\; M\)
- \([CO_2]_f =[CO_2]_i+Δ[CO_2]=(0.0150−0.00369)\; M=0.0113\; M\)
- \([H_2O]_f=[H_2O]_i+Δ[H_2O]=(0+0.00369) \;M=0.00369\; M\)
- \([CO]_f=[CO]_i+Δ[CO]=(0+0.00369)\; M=0.00369 \;M\)
We can check our work by inserting the calculated values back into the equilibrium constant expression:
\[K=\dfrac{[H_2O][CO]}{[H_2][CO_2]}=\dfrac{(0.00369)^2}{(0.0113)^2}=0.107\]
To two significant figures, this K is the same as the value given in the problem, so our answer is confirmed.
Exercise
Hydrogen gas reacts with iodine vapor to give hydrogen iodide according to the following chemical equation:
\[H_{2(g)}+I_{2(g)} \rightleftharpoons 2HI_{(g)}\]
K = 54 at 425°C. If 0.172 M H2 and I2 are injected into a reactor and maintained at 425°C until the system equilibrates, what is the final concentration of each substance in the reaction mixture?
Answer: [HI]f = 0.270 M; [H2]f = [I2]f = 0.037 M
In Example 14.3.3, the initial concentrations of the reactants were the same, which gave us an equation that was a perfect square and simplified our calculations. Often, however, the initial concentrations of the reactants are not the same, and/or one or more of the products may be present when the reaction starts. Under these conditions, there is usually no way to simplify the problem, and we must determine the equilibrium concentrations with other means. Such a case is described in Example 14.3.4.
Example 14.3.4
In the water–gas shift reaction shown in Example 14.3.3, a sample containing 0.632 M CO2 and 0.570 M H2 is allowed to equilibrate at 700 K. At this temperature, K = 0.106. What is the composition of the reaction mixture at equilibrium?
Given: balanced equilibrium equation, concentrations of reactants, and K
Asked for: composition of reaction mixture at equilibrium
Strategy:
A Write the equilibrium equation. Construct a table showing the initial concentrations of all substances in the mixture. Complete the table showing the changes in the concentrations (x) and the final concentrations.
B Write the equilibrium constant expression for the reaction. Substitute the known K value and the final concentrations to solve for x.
C Calculate the final concentration of each substance in the reaction mixture. Check your answers by substituting these values into the equilibrium constant expression to obtain K.
Solution:
A [CO2]i = 0.632 M and [H2]i = 0.570 M. Again, x is defined as the change in the concentration of H2O: Δ[H2O] = +x. Because 1 mol of CO is produced for every 1 mol of H2O, the change in the concentration of CO is the same as the change in the concentration of H2O, so Δ[CO] = +x. Similarly, because 1 mol each of H2 and CO2 are consumed for every 1 mol of H2O produced, Δ[H2] = Δ[CO2] = −x. The final concentrations are the sums of the initial concentrations and the changes in concentrations at equilibrium.
|
\[H_{2(g)}+CO_{2(g)} \rightleftharpoons H_2O_{(g)}+CO_{(g)}\] |
||||
|---|---|---|---|---|
| [H2] | [CO2] | [H2O] | [CO] | |
| initial | 0.570 | 0.632 | 0 | 0 |
| change | −x | −x | +x | +x |
| final | (0.570 − x) | (0.632 − x) | x | x |
B We can now use the equilibrium equation and the known K value to solve for x:
\[K=\dfrac{[H_2O][CO]}{[H_2][CO_2]}=\dfrac{x^2}{(0.570−x)(0.632−x)}=0.106\]
In contrast to Example 10, however, there is no obvious way to simplify this expression. Thus we must expand the expression and multiply both sides by the denominator:
\[x^2 = 0.106(0.360 − 1.20x + x^2)\]
Collecting terms on one side of the equation,
\[0.894x^2 + 0.127x − 0.0382 = 0\]
This equation can be solved using the quadratic formula:
\[ x = \dfrac{-b \pm \sqrt{b^2-4ac}}{2a} = \dfrac{−0.127 \pm \sqrt{(0.127)^2−4(0.894)(−0.0382)}}{2(0.894)}\]
\[x =0.148 \text{ and } −0.290\]
Only the answer with the positive value has any physical significance, so Δ[H2O] = Δ[CO] = +0.148 M, and Δ[H2] = Δ[CO2] = −0.148 M.
C The final concentrations of all species in the reaction mixture are as follows:
- \([H_2]_f[ = [H_2]_i+Δ[H_2]=0.570 \;M −0.148\; M=0.422 M\)
- \([CO_2]_f =[CO_2]_i+Δ[CO_2]=0.632 \;M−0.148 \;M=0.484 M\)
- \([H_2O]_f =[H_2O]_i+Δ[H_2O]=0\; M+0.148\; M =0.148\; M\)
- \([CO]_f=[CO]_i+Δ[CO]=0 M+0.148\;M=0.148 M\)
We can check our work by substituting these values into the equilibrium constant expression:
\[K=\dfrac{[H_2O][CO]}{[H_2][CO_2]}=\dfrac{(0.148)^2}{(0.422)(0.484)}=0.107\]
Because K is essentially the same as the value given in the problem, our calculations are confirmed.
Exercise
The exercise in Example 8 showed the reaction of hydrogen and iodine vapor to form hydrogen iodide, for which K = 54 at 425°C. If a sample containing 0.200 M H2 and 0.0450 M I2 is allowed to equilibrate at 425°C, what is the final concentration of each substance in the reaction mixture?
Answer: [HI]f = 0.0882 M; [H2]f = 0.156 M; [I2]f = 9.2 × 10−4 M
In many situations it is not necessary to solve a quadratic (or higher-order) equation. Most of these cases involve reactions for which the equilibrium constant is either very small (K ≤ 10−3) or very large (K ≥ 103), which means that the change in the concentration (defined as x) is essentially negligible compared with the initial concentration of a substance. Knowing this simplifies the calculations dramatically, as illustrated in Example 14.3.5.
Example 14.3.5
Atmospheric nitrogen and oxygen react to form nitric oxide:
\[N_{2(g)}+O_{2(g)} \rightleftharpoons 2NO_{(g)}\]
Kp = 2.0 × 10−31 at 25°C. What is the partial pressure of NO in equilibrium with N2 and O2 in the atmosphere (at 1 atm, P{N2} = 0.78 atm and P{O2} = 0.21 atm
Given: balanced equilibrium equation and values of Kp, P{O2} and P{N2}
Asked for: partial pressure of NO
Strategy:
A Construct a table and enter the initial partial pressures, the changes in the partial pressures that occur during the course of the reaction, and the final partial pressures of all substances.
B Write the equilibrium equation for the reaction. Then substitute values from the table to solve for the change in concentration (x).
C Calculate the partial pressure of NO. Check your answer by substituting values into the equilibrium equation and solving for K.
Solution:
A Because we are given Kp and partial pressures are reported in atmospheres, we will use partial pressures. The initial partial pressure of O2 is 0.21 atm and that of N2 is 0.78 atm. If we define the change in the partial pressure of NO as 2x, then the change in the partial pressure of O2 and of N2 is −x because 1 mol each of N2 and of O2 is consumed for every 2 mol of NO produced. Each substance has a final partial pressure equal to the sum of the initial pressure and the change in that pressure at equilibrium.
|
\[N_{2(g)}+O_{2(g)} \rightleftharpoons 2NO_{(g)}\] |
|||
|---|---|---|---|
| P{N2} | P{N2} | P{NO} | |
| initial P | 0.78 | 0.21 | 0 |
| change in P | −x | −x | +2x |
| final P | (0.78 − x) | (0.21 − x) | 2x |
B Substituting these values into the equation for the equilibrium constant,
\[K_p=\dfrac{(P_{NO})^2}{(P_{N_2})(P_{O_2})}=\dfrac{(2x)^2}{(0.78−x)(0.21−x)}=2.0 \times 10^{−31}\]
In principle, we could multiply out the terms in the denominator, rearrange, and solve the resulting quadratic equation. In practice, it is far easier to recognize that an equilibrium constant of this magnitude means that the extent of the reaction will be very small; therefore, the x value will be negligible compared with the initial concentrations. If this assumption is correct, then to two significant figures, (0.78 − x) = 0.78 and (0.21 − x) = 0.21. Substituting these expressions into our original equation,
\[\dfrac{(2x)^2}{(0.78)(0.21)} = 2.0 \times 10^{−31}\]
\[\dfrac{4x^2}{0.16} =2.0 \times10^{−31}\]
\[x^2=\dfrac{0.33 \times 10^{−31}}{4}\]
\[x^=9.1 \times 10^{−17}\]
C Substituting this value of x into our expressions for the final partial pressures of the substances,
- \(P_{NO}=2x \; atm=1.8 \times 10^{−16} \;atm \)
- \(P_{N_2}=(0.78−x) \;atm=0.78 \;atm \)
- \(P_{O_2}=(0.21−x) \;atm=0.21\; atm\)
From these calculations, we see that our initial assumption regarding x was correct: given two significant figures, 2.0 × 10−16 is certainly negligible compared with 0.78 and 0.21. When can we make such an assumption? As a general rule, if x is less than about 5% of the total, or 10−3 > K > 103, then the assumption is justified. Otherwise, we must use the quadratic formula or some other approach. The results we have obtained agree with the general observation that toxic NO, an ingredient of smog, does not form from atmospheric concentrations of N2 and O2 to a substantial degree at 25°C. We can verify our results by substituting them into the original equilibrium equation:
\[K_p=\dfrac{(P_{NO})^2}{(P_{N_2})(P_{O_2})}=\dfrac{(1.8 \times 10^{−16})^2}{(0.78)(0.21)}=2.0 times 10^{−31}\]
The final Kp agrees with the value given at the beginning of this example.
Exercise
Under certain conditions, oxygen will react to form ozone, as shown in the following equation:
\[H_{2(g)}+C_2H_{4(g)} \overset{Ni}{\rightleftharpoons} C_2H_{6(g)}\]
Kp = 2.5 × 10−59 at 25°C. What ozone partial pressure is in equilibrium with oxygen in the atmosphere P(O2) =0.21 atm?
Answer: 4.8 × 10−31 atm
Another type of problem that can be simplified by assuming that changes in concentration are negligible is one in which the equilibrium constant is very large (K ≥ 103). A large equilibrium constant implies that the reactants are converted almost entirely to products, so we can assume that the reaction proceeds 100% to completion. When we solve this type of problem, we view the system as equilibrating from the products side of the reaction rather than the reactants side. This approach is illustrated in Example 14.3.6.
Example 14.3.6
The chemical equation for the reaction of hydrogen with ethylene (C2H4) to give ethane (C2H6) is as follows:
\[H_{2(g)}+C_2H_{4(g)} \overset{Ni}{\rightleftharpoons} C_2H_{6(g)}\]
K = 9.6 × 1018 at 25°C. If a mixture of 0.200 M H2 and 0.155 M C2H4 is maintained at 25°C in the presence of a powdered nickel catalyst, what is the equilibrium concentration of each substance in the mixture?
Given: balanced chemical equation, K, and initial concentrations of reactants
Asked for: equilibrium concentrations
Strategy:
A Construct a table showing initial concentrations, concentrations that would be present if the reaction were to go to completion, changes in concentrations, and final concentrations.
B Write the equilibrium constant expression for the reaction. Then substitute values from the table into the expression to solve for x (the change in concentration).
C Calculate the equilibrium concentrations. Check your answers by substituting these values into the equilibrium equation.
Solution:
A From the magnitude of the equilibrium constant, we see that the reaction goes essentially to completion. Because the initial concentration of ethylene (0.155 M) is less than the concentration of hydrogen (0.200 M), ethylene is the limiting reactant; that is, no more than 0.155 M ethane can be formed from 0.155 M ethylene. If the reaction were to go to completion, the concentration of ethane would be 0.155 M and the concentration of ethylene would be 0 M. Because the concentration of hydrogen is greater than what is needed for complete reaction, the concentration of unreacted hydrogen in the reaction mixture would be 0.200 M − 0.155 M = 0.045 M. The equilibrium constant for the forward reaction is very large, so the equilibrium constant for the reverse reaction must be very small. The problem then is identical to that in Example 12. If we define −x as the change in the ethane concentration for the reverse reaction, then the change in the ethylene and hydrogen concentrations is +x. The final equilibrium concentrations are the sums of the concentrations for the forward and reverse reactions.
|
\[H_{2(g)}+C_2H_{4(g)} \overset{Ni}{\rightleftharpoons} C_2H_{6(g)}\] |
|||
|---|---|---|---|
| [H2] | [C2H4] | [C2H6] | |
| initial | 0.200 | 0.155 | 0 |
| assuming 100% reaction | 0.045 | 0 | 0.155 |
| change | +x | +x | −x |
| final | (0.045 + x) | (0 + x) | (0.155 − x) |
B Substituting values into the equilibrium constant expression,
\[K=\dfrac{[C_2H_6]}{[H_2][C_2H_4]}=\dfrac{0.155−x}{(0.045+x)x}=9.6 \times 10^{18}\]
Once again, the magnitude of the equilibrium constant tells us that the equilibrium will lie far to the right as written, so the reverse reaction is negligible. Thus x is likely to be very small compared with either 0.155 M or 0.045 M, and the equation can be simplified [(0.045 + x) = 0.045 and (0.155 − x) = 0.155] as follows:
\[K=\dfrac{0.155}{0.045x} = 9.6 \times 10^{18}\]
\[x=3.6 \times 10^{−19}\]
C The small x value indicates that our assumption concerning the reverse reaction is correct, and we can therefore calculate the final concentrations by evaluating the expressions from the last line of the table:
- \([C_2H_6]_f = (0.155 − x)\; M = 0.155 \; M\)
- \([C_2H_4]_f = x\; M = 3.6 \times 10^{−19} M \)
- \([H_2]_f = (0.045 + x) \;M = 0.045 \;M\)
We can verify our calculations by substituting the final concentrations into the equilibrium constant expression:
\[K=\dfrac{[C_2H_6]}{[H_2][C_2H_4]}=\dfrac{0.155}{(0.045)(3.6 \times 10^{−19})}=9.6 \times 10^{18}\]
This K value agrees with our initial value at the beginning of the example.
Exercise
Hydrogen reacts with chlorine gas to form hydrogen chloride:
\[H_{2(g)}+Cl_{2(g)} \rightleftharpoons 2HCl_{(g)}\]
Kp = 4.0 × 1031 at 47°C. If a mixture of 0.257 M H2 and 0.392 M Cl2 is allowed to equilibrate at 47°C, what is the equilibrium composition of the mixture?
Answer:
- \([H_2]_f = 4.8 \times 10^{−32}\; M\)
- \([Cl_2]_f = 0.135\; M\)
- \([HCl]_f = 0.514\; M\)
Summary
When an equilibrium constant is calculated from equilibrium concentrations, molar concentrations or partial pressures are substituted into the equilibrium constant expression for the reaction. Equilibrium constants can be used to calculate the equilibrium concentrations of reactants and products by using the quantities or concentrations of the reactants, the stoichiometry of the balanced chemical equation for the reaction, and a tabular format to obtain the final concentrations of all species at equilibrium.
Key Takeaway
- Various methods can be used to solve the two fundamental types of equilibrium problems: (1) those in which we calculate the concentrations of reactants and products at equilibrium and (2) those in which we use the equilibrium constant and the initial concentrations of reactants to determine the composition of the equilibrium mixture.
Conceptual Problems
-
Describe how to determine the magnitude of the equilibrium constant for a reaction when not all concentrations of the substances are known.
-
Calculations involving systems with very small or very large equilibrium constants can be dramatically simplified by making certain assumptions about the concentrations of products and reactants. What are these assumptions when K is (a) very large and (b) very small? Illustrate this technique using the system A + 2B ⇌ C for which you are to calculate the concentration of the product at equilibrium starting with only A and B. Under what circumstances should simplifying assumptions not be used?
Numerical Problems
-
In the equilibrium reaction A + B ⇌C, what happens to K if the concentrations of the reactants are doubled? tripled? Can the same be said about the equilibrium reaction A ⇌B + C?
-
The following table shows the reported values of the equilibrium P{O2} at three temperatures for the reaction Ag2O(s) ⇌ 2 Ag(s) + 1/2 O2(g) for which ΔH° = 31 kJ/mol. Are these data consistent with what you would expect to occur? Why or why not?
T (°C) P(O2) mm Hg 150 182 184 143 191 126 -
Given the equilibrium system N2O4(g) ⇌ 2 NO2(g), what happens to Kp if the initial pressure of N2O4 is doubled? If Kp is 1.7 × 10−1 at 2300°C, and the system initially contains 100% N2O4 at a pressure of 2.6 × 102 atm, what is the equilibrium pressure of each component?
-
At 430°C, 4.20 mol of HI in a 9.60 L reaction vessel reaches equilibrium according to the following equation: H2(g) + I2(g) ⇌2HI(g) At equilibrium, [H2] = 0.047 M and [HI] = 0.345 M. What are K and Kp for this reaction?
-
Methanol, a liquid used as an automobile fuel additive, is commercially produced from carbon monoxide and hydrogen at 300°C according to the following reaction: CO(g) + 2H2(g) ⇌ CH3OH(g) and Kp = 1.3 × 10−4. If 56.0 g of CO is mixed with excess hydrogen in a 250 mL flask at this temperature, and the hydrogen pressure is continuously maintained at 100 atm, what would be the maximum percent yield of methanol? What pressure of hydrogen would be required to obtain a minimum yield of methanol of 95% under these conditions?
-
Starting with pure A, if the total equilibrium pressure is 0.969 atm for the reactionA(s ⇌ 2 B(g) + C(g), what is Kp?
-
The decomposition of ammonium carbamate to NH3 and CO2 at 40°C is written as NH4CO2NH2(s) ⇌ 2NH3(g) + CO2 If the partial pressure of NH3 at equilibrium is 0.242 atm, what is the equilibrium partial pressure of CO2? What is the total gas pressure of the system? What is Kp?
-
At 375 K, Kp for the reaction SO2Cl2(g) ⇌ SO2(g) + Cl2g) is 2.4, with pressures expressed in atmospheres. At 303 K, Kp is 2.9 × 10−2.
- What is K for the reaction at each temperature?
- If a sample at 375 K has 0.100 M Cl2 and 0.200 M SO2 at equilibrium, what is the concentration of SO2Cl2?
- If the sample given in part b is cooled to 303 K, what is the pressure inside the bulb?
-
For the gas-phase reaction aA ⇌ bB, show that Kp = K(RT)Δn assuming ideal gas behavior.
-
For the gas-phase reaction I2 ⇌2I, show that the total pressure is related to the equilibrium pressure by the following equation:
\[P_T=\sqrt{K_pP_{I_2}} + P_{I_2}\]
-
Experimental data on the system Br2(l) ⇌ Br2(aq) are given in the following table. Graph [Br2] versus moles of Br2(l) present; then write the equilibrium constant expression and determine K.
Grams Br2 in 100 mL Water [Br2] (M) 1.0 0.0626 2.5 0.156 3.0 0.188 4.0 0.219 4.5 0.219 -
Data accumulated for the reaction n-butane(g) ⇌ isobutane(g) at equilibrium are shown in the following table. What is the equilibrium constant for this conversion? If 1 mol of n-butane is allowed to equilibrate under the same reaction conditions, what is the final number of moles of n-butane and isobutane?
Moles n-butane Moles Isobutane 0.5 1.25 1.0 2.5 1.50 3.75 -
Solid ammonium carbamate (NH4CO2NH2) dissociates completely to ammonia and carbon dioxide when it vaporizes:
[NH_4CO_2NH_{2(s)} \rightleftharpoons 2NH_{3(g)}+CO_{2(g)}\]
At 25°C, the total pressure of the gases in equilibrium with the solid is 0.116 atm. What is the equilibrium partial pressure of each gas? What is Kp? If the concentration of CO2 is doubled and then equilibrates to its initial equilibrium partial pressure +x atm, what change in the NH3 concentration is necessary for the system to restore equilibrium?
-
The equilibrium constant for the reaction COCl2(g) ⇌ CO(g) + Cl2(g) is Kp = 2.2 × 10−10 at 100°C. If the initial concentration of COCl2 is 3.05 × 10−3 M, what is the partial pressure of each gas at equilibrium at 100°C? What assumption can be made to simplify your calculations?
-
Aqueous dilution of IO4− results in the following reaction:
\[IO^−_{4(aq)}+2H_2O_{(l)} \rightleftharpoons H_4IO^−_{6(aq)}\]
and K = 3.5 × 10−2. If you begin with 50 mL of a 0.896 M solution of IO4− that is diluted to 250 mL with water, how many moles of H4IO6− are formed at equilibrium?
-
Iodine and bromine react to form IBr, which then sublimes. At 184.4°C, the overall reaction proceeds according to the following equation:
\[I_{2(g)}+Br_{2(g)} \rightleftharpoons 2IBr_{(g)}\]
Kp = 1.2 × 102. If you begin the reaction with 7.4 g of I2 vapor and 6.3 g of Br2 vapor in a 1.00 L container, what is the concentration of IBr(g) at equilibrium? What is the partial pressure of each gas at equilibrium? What is the total pressure of the system?
-
For the reaction
\[C_{(s)} + 12N_{2(g)}+\frac{5}{2}H_{2(g)} \rightleftharpoons CH3NH2(g)\]
K = 1.8 × 10−6. If you begin the reaction with 1.0 mol of N2, 2.0 mol of H2, and sufficient C(s) in a 2.00 L container, what are the concentrations of N2 and CH3NH2 at equilibrium? What happens to K if the concentration of H2 is doubled?
Contributors
- Anonymous
Modified by Joshua Halpern (Howard University), Scott Sinex, and Scott Johnson (PGCC)


