4.7: Separating Mixtures through Physcial Changes
- Page ID
- 365763
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
There are many techniques that use physical changes to separate mixtures. Here are a number of common separation techniques:
Distillation
Distillation is an effective method to separate mixtures comprised of two or more pure liquids. Distillation is a purification process where one component of a liquid mixture is vaporized and then condensed and isolated. In simple distillation Figure \(\PageIndex{1}\), a mixture is heated and the most volatile component vaporizes at the lowest temperature. The vapor passes through a cooled tube (a condenser), where it condenses back into its liquid state. The condensate that is collected is called distillate.
Outside the chemistry lab, distillation is commonly used to increase the alcohol content of liquors such as vodka, whiskey, and brandy.
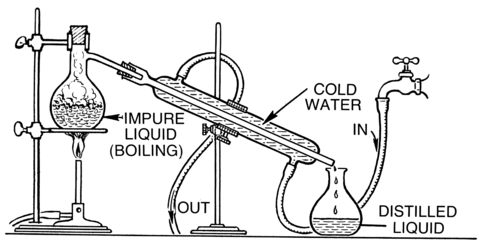
Figure \(\PageIndex{1}\) Distillation apparatus.
Evaporation
Evaporation is a technique used to separate out a soluble solid from a liquid. The method drives off the liquid components from the solid components. The process typically involves heating the mixture until no more liquid remains. Evaporation can be used, for example, to obtain table salt from sea water, Figure \(\PageIndex{2}\). The heat for the process comes from the sun.

Figure \(\PageIndex{2}\) Once the sea water in these evaporation ponds has evaporated, the salt can be harvested.
Filtration
Filtration is a separation method used to separate out pure substances in mixtures comprised of particles, some of which are large enough in size to be captured with a porous material. Particle size can vary considerably, given the type of mixture. For instance, stream water is a mixture that contains naturally occurring biological organisms like bacteria, viruses, and protozoans. Some water filters can filter out bacteria, the length of which is on the order of 1 micron. Other mixtures, like soil, have relatively large particle sizes, which can be filtered through something like a coffee filter.
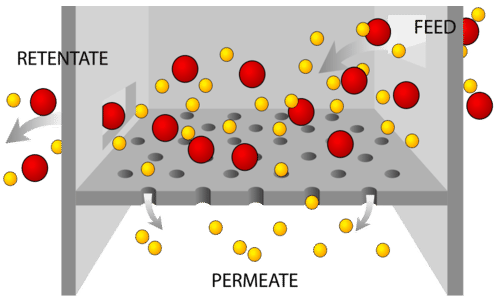
Figure \(\PageIndex{3}\) Filtration.
Chromatography
Chromatography is the separation of a liquid or gas mixture by passing it through a medium in which the components move at different rates. Thin-layer chromatography is perhaps the simplist form of chromatography. It uses a plate--a plastic or glass slide covered on one side with silica (essentially very fine, purified sand), alumina, or some other solid. The plate is placed so that the bottom of it is submerged in a solvent, and the solvent then moves upward on the plate. If a drop of a mixture is placed a little above the level of the solvent, different components of the mixture will be carried upward at different rates depending on how strongly they interact with the silica. Figure \(\PageIndex{4}\) shows separation of the chromophores (colored compounds) in a spinach leaf by thin layer chromatography.The pencil line at the bottom of the plate shows the original position of the mixture before the solvent started traveling up the plate.
Figure \(\PageIndex{4}\) Separation of compounds in a spinch leaf. (photo credit: Heather Coleman)


