3.6: Temperature
- Page ID
- 365759
- Convert temperatures between Celsius, Kelvin, and Fahrenheit scales.
The concept of temperature may seem familiar to you, but many people confuse temperature with heat. Temperature is a measure of the average kinetic energy of the particles in a sample of matter. As temperature increases, the kinetic energy of the particles also increases. Heat, on the other hand, is the flow of thermal energy from an object with a higher temperature to an object with a lower temperature. Temperature is an important parameter in chemistry. When a substance changes from solid to liquid, it is almost always because there was an increase in the temperature of the material. Chemical reactions usually proceed faster if the temperature is increased. Many unstable materials (such as enzymes) will be viable longer at lower temperatures.

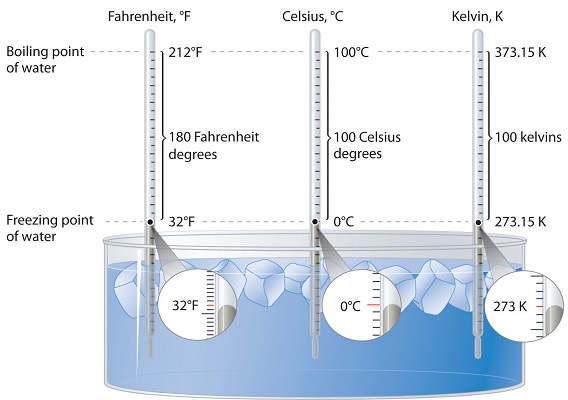
Three different scales are commonly used to measure temperature: Fahrenheit (expressed as °F), Celsius (°C), and Kelvin (K). Thermometers measure temperature by using materials that expand or contract when heated or cooled. Mercury or alcohol thermometers, for example, have a reservoir of liquid that expands when heated and contracts when cooled, so the liquid column lengthens or shortens as the temperature of the liquid changes.
The Fahrenheit Scale
The main problem with the Fahrenheit scale is the arbitrary definitions of temperature. The freezing point of water was defined as \(32^\text{o} \text{F}\) and the boiling point as \(212^\text{o} \text{F}\). The Fahrenheit scale is typically not used for scientific purposes.
The Celsius Scale
The Celsius scale sets the freezing point and boiling point of water at \(0^\text{o} \text{C}\) and \(100^\text{o} \text{C}\) respectively. The distance between those two points is divided into 100 equal intervals, each of which is one degree. Another term sometimes used for the Celsius scale is "centigrade" because there are 100 degrees between the freezing and boiling points of water on this scale. However, the preferred term is "Celsius".
The Kelvin Scale
The Kelvin temperature scale is based on molecular motion, with the temperature of \(0 \: \text{K}\), also known as absolute zero, being the point where all molecular motion ceases. The freezing point of water on the Kelvin scale is \(273.15 \: \text{K}\), while the boiling point is \(373.15 \: \text{K}\). Notice that there is no "degree" used in the temperature designation. Unlike the Fahrenheit and Celsius scales where temperatures are referred to as "degrees \(\text{F}\)" or "degrees \(\text{C}\)", we simply designate temperatures in the Kelvin scale as Kelvins.
Converting Between Scales
The Kelvin is the same size as the Celsius degree, so measurements are easily converted from one to the other. The freezing point of water is 0°C = 273.15 K; the boiling point of water is 100°C = 373.15 K. The Kelvin and Celsius scales are related as follows:
\[T \,\text{(in °C)} + 273.15 = T \, \text{(in K)} \tag{3.10.1} \label{3.10.1}\]
\[T \; \text{ (in K)} − 273.15 = T \; \text{(in °C)} \tag{3.10.2} \label{3.10.2} \]
Degrees on the Fahrenheit scale, however, are based on an English tradition of using 12 divisions, just as 1 ft = 12 in. The relationship between degrees Fahrenheit and degrees Celsius is as follows:
\[°C = \dfrac{(°F-32)}{1.8} \tag{3.10.3} \label{3.10.3}\]
\[°F = 1.8 \times (°C)+32 \tag{3.10.4} \label{3.10.4} \]
A student is ill with a temperature of 103.5°F. What is her temperature in °C and K?
Solution
Converting from Fahrenheit to Celsius requires the use of Equation \ref{3.10.3}:
\[\begin{align} °C &= \dfrac{(103.5°F - 32)}{1.8} \\ &= 39.7 \,°C \end{align}\]
Converting from Celsius to Kelvin requires the use of Equation \ref{3.10.1}:
\[\begin{align} K &= 39.7 \,°C + 273.15 \\ &= 312.9\,K \end{align}\]
Convert each temperature to °C and °F.
- the temperature of the surface of the sun (5800 K)
- the boiling point of gold (3080 K)
- the boiling point of liquid nitrogen (77.36 K)
- Answer (a)
- 5527 °C, 9981 °F
- Answer (b)
- 2807 °C, 5085 °F
- Answer (c)
- -195.79 °C, -320.42 °F
Contributions & Attributions
This page was constructed from content via the following contributor(s) and edited (topically or extensively) by the LibreTexts development team to meet platform style, presentation, and quality:
Henry Agnew (UC Davis)

