3.2: Multi-Step Conversion Problems
- Page ID
- 365756
- Given a quantity, convert from one set of units to another using dimensional analysis showing canceling on units. This includes multiple factor conversions.
Multiple Conversions
Sometimes you will have to perform more than one conversion to obtain the desired unit. For example, suppose you want to convert 54.7 km into millimeters. We will set up a series of conversion factors so that each conversion factor produces the next unit in the sequence. We first convert the given amount in km to the base unit, which is meters. We know that 1,000 m =1 km.
Then we convert meters to mm, remembering that \(1\; \rm{mm}\) = \( 10^{-3}\; \rm{m}\).
Concept Map
Calculation
\[ \begin{align*} 54.7 \; \cancel{\rm{km}} \times \dfrac{1,000 \; \cancel{\rm{m}}}{1\; \cancel{\rm{km}}} \times \dfrac{1\; \rm{mm}}{10^{-3}\cancel{ \rm{m}}} & = 54,700,000 \; \rm{mm} \\ &= 5.47 \times 10^7\; \rm{mm} \end{align*}\]
In each step, the previous unit is canceled and the next unit in the sequence is produced, each successive unit canceling out until only the unit needed in the answer is left.
Convert 58.2 ms to megaseconds in one multi-step calculation.
Solution
|
Steps for Problem Solving |
Unit Conversion |
|---|---|
|
Identify the "given" information and what the problem is asking you to "find." |
Given: 58.2 ms Find: Ms |
| List other known quantities |
\(1 ms = 10^{-3} s \) \(1 Ms = 10^6s \) |
|
Prepare a concept map. |
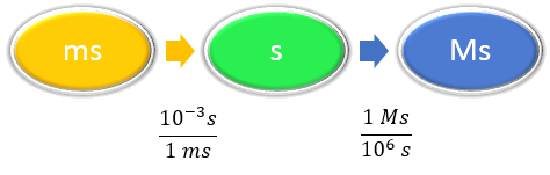 |
|
Calculate. |
\[ \begin{align} 58.2 \; \cancel{\rm{ms}} \times \dfrac{10^{-3} \cancel{\rm{s}}}{1\; \cancel{\rm{ms}}} \times \dfrac{1\; \rm{Ms}}{1,000,000\; \cancel{ \rm{s}}} & =0.0000000582\; \rm{Ms} \nonumber\\ &= 5.82 \times 10^{-8}\; \rm{Ms}\nonumber \end{align}\nonumber \] Neither conversion factor affects the number of significant figures in the final answer. |
How many seconds are in 2.50 days?
Solution
|
Steps for Problem Solving |
Unit Conversion |
|---|---|
|
Identify the "given" information and what the problem is asking you to "find." |
Given: 2.50 days Find: s |
|
List other known quantities. |
1 day = 24 hours 1 hour = 60 minutes 1 minute = 60 seconds |
|
Prepare a concept map. |
|
|
Calculate. |
\[2.50 \: \text{d} \times \frac{24 \: \text{hr}}{1 \: \text{d}}\times \frac{60 \: \text{min}}{1 \: \text{hr}} \times \frac{60 \: \text{s}}{1 \: \text{min}} = 216,000 \: \text{s} \nonumber\] 2.16 x 105 s 3SF, not ambiguous |
Perform each conversion in one multi-step calculation.
- 43.007 ng to kg
- 1005 in to yd
- Answer a
- \(4.3007 \times 10^{-11} kg \)
- Answer b
- \(27.92\, yd\)
Contributions
Henry Agnew (UC Davis)

