2.5: The International System of Units
- Page ID
- 365754
- List SI units of length, mass, time, and temperature.
- List the multipliers, symbols, and numerical meanings for all SI units.
People who live in the United States measure weight in pounds, height in feet and inches, and a car’s speed in miles per hour. In contrast, chemistry and other branches of science use the International System of Units (also known as SI after Système Internationale d’Unités), which was established so that scientists around the world could communicate efficiently with each other. Many countries have also adopted SI units for everyday use as well. The United States is one of the few countries that has not.
Base SI Units
Base (or basic) units, are the fundamental units of SI. There are seven base units, which are listed in Table \(\PageIndex{1}\), Chemistry uses five of the base units: the mole for amount, the kilogram for mass, the meter for length, the second for time, and the Kelvin for temperature. The degree Celsius (°C) is also commonly used for temperature. The numerical relationship between Kelvins and degrees Celsius is as follows:
\[K = °C + 273 \label{Eq1}\]
| Property | Unit | Abbreviation |
|---|---|---|
| length | meter | m |
| mass | kilogram | kg |
| time | second | s |
| amount | mole | mol |
| temperature | Kelvin | K |
| electrical current | ampere | amp |
| luminous intensity | candela | cd |
The United States uses the English (sometimes called Imperial) system of units for many quantities. Inches, feet, miles, gallons, pounds, and so forth, are all units connected with the English system of units. There have been many mistakes due to the improper conversion of units between the SI and English systems.
The size of each base unit is defined by international convention. For example, the kilogram is defined as the quantity of mass of a special metal cylinder kept in a vault in France (Figure \(\PageIndex{1}\)). The other base units have similar definitions and standards. The sizes of the base units are not always convenient for all measurements. For example, a meter is a rather large unit for describing the width of something as narrow as human hair. Instead of reporting the diameter of hair as 0.00012 m or as 1.2 × 10−4 m using scientific notation as discussed in section 1.4, SI also provides a series of prefixes that can be attached to the units, creating units that are larger or smaller by powers of 10.

Common prefixes and their multiplicative factors are listed in Table \(\PageIndex{2}\). (Perhaps you have already noticed that the base unit kilogram is a combination of a prefix, kilo- meaning 1,000 ×, and a unit of mass, the gram.) Some prefixes create a multiple of the original unit: 1 kilogram equals 1,000 grams, and 1 megameter equals 1,000,000 meters. Other prefixes create a fraction of the original unit. Thus, 1 centimeter equals 1/100 of a meter, 1 millimeter equals 1/1,000 of a meter, 1 microgram equals 1/1,000,000 of a gram, and so forth.
| Prefix | Abbreviation | Multiplicative Factor | Multiplicative Factor in Scientific Notation |
|---|---|---|---|
| giga- | G | 1,000,000,000 × | 109 × |
| mega- | M | 1,000,000 × | 106 × |
| kilo- | k | 1,000 × | 103 × |
| deca- | D | 10 × | 101 × |
| deci- | d | 1/10 × | 10−1 × |
| centi- | c | 1/100 × | 10−2 × |
| milli- | m | 1/1,000 × | 10−3 × |
| micro- | µ* | 1/1,000,000 × | 10−6 × |
| nano- | n | 1/1,000,000,000 × | 10−9 × |
| *The letter µ is the Greek lowercase letter for m and is called “mu,” which is pronounced “myoo.” | |||
Both SI units and prefixes have abbreviations, and the combination of a prefix abbreviation with a base unit abbreviation gives the abbreviation for the modified unit. For example, kg is the abbreviation for kilogram. We will be using these abbreviations throughout this book.
The mass of a body is a measure of its inertial property or how much matter it contains. The weight of a body is a measure of the force exerted on it by gravity or the force needed to support it. Gravity on earth gives a body a downward acceleration of about 9.8 m/s2. In common parlance, weight is often used as a synonym for mass in weights and measures. For instance, the verb “to weigh” means “to determine the mass of” or “to have a mass of.” The incorrect use of weight in place of mass should be phased out, and the term mass used when mass is meant. The SI unit of mass is the kilogram (kg). In science and technology, the weight of a body in a particular reference frame is defined as the force that gives the body an acceleration equal to the local acceleration of free fall in that reference frame. Thus, the SI unit of the quantity weight defined in this way (force) is the newton (N).
Derived SI Units
Derived units are combinations of SI base units. Units can be multiplied and divided, just as numbers can be multiplied and divided. For example, the area of a square having a side of 2 cm is 2 cm × 2 cm, or 4 cm2 (read as “four centimeters squared” or “four square centimeters”). Notice that we have squared a length unit, the centimeter, to get a derived unit for area, the square centimeter.
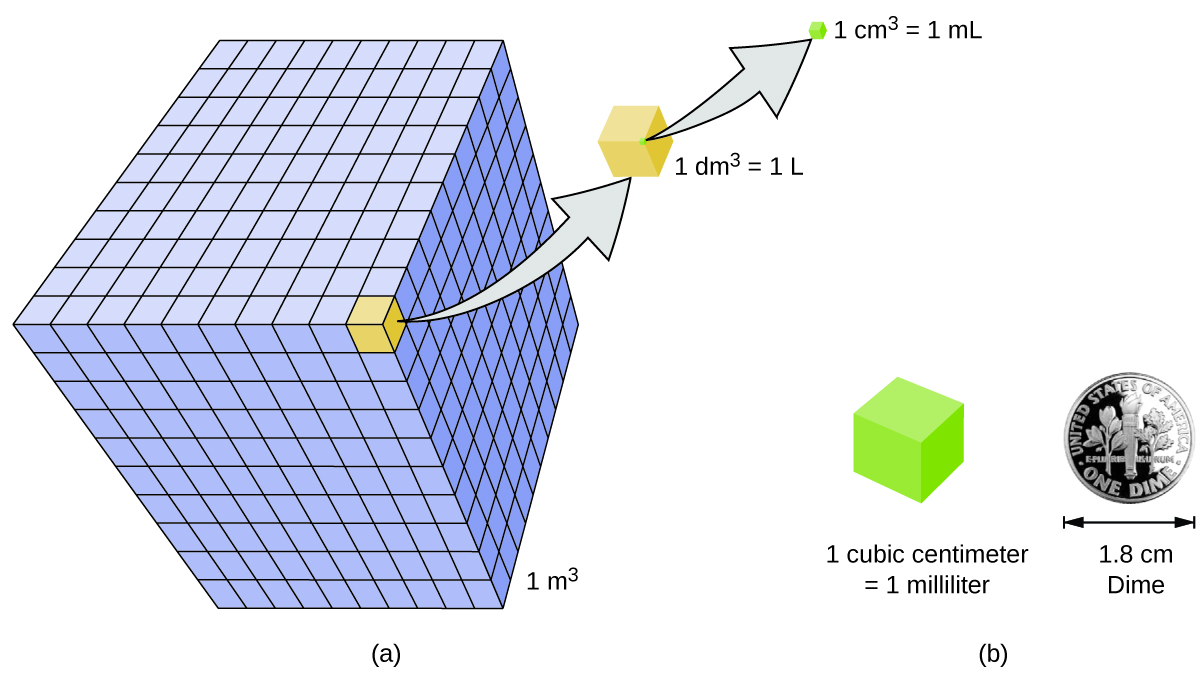
Volume is an important quantity that uses a derived unit. Volume is the amount of space that a given substance occupies and is defined geometrically as length × width × height. Each distance can be expressed using the meter unit, so volume has the derived unit m × m × m, or m3 (read as “meters cubed” or “cubic meters”). A cubic meter is a rather large volume, so scientists typically express volumes in terms of 1/1,000 of a cubic meter. This unit has its own name—the liter (L). A liter is a little larger than 1 US quart in volume. Below are approximate equivalents for some of the units used in chemistry.
Approximate Equivalents to Some SI Units
- 1 m ≈ 39.36 in. ≈ 3.28 ft ≈ 1.09 yd
- 1 in. = 2.54 cm (this is exact rather than an approximation)
- 1 km ≈ 0.6214 mi
- 1 kg ≈ 2.205 lb
- 1 lb ≈ 454 g
- 1 L ≈ 1.06 qt
- 1 qt ≈ 0.946 L
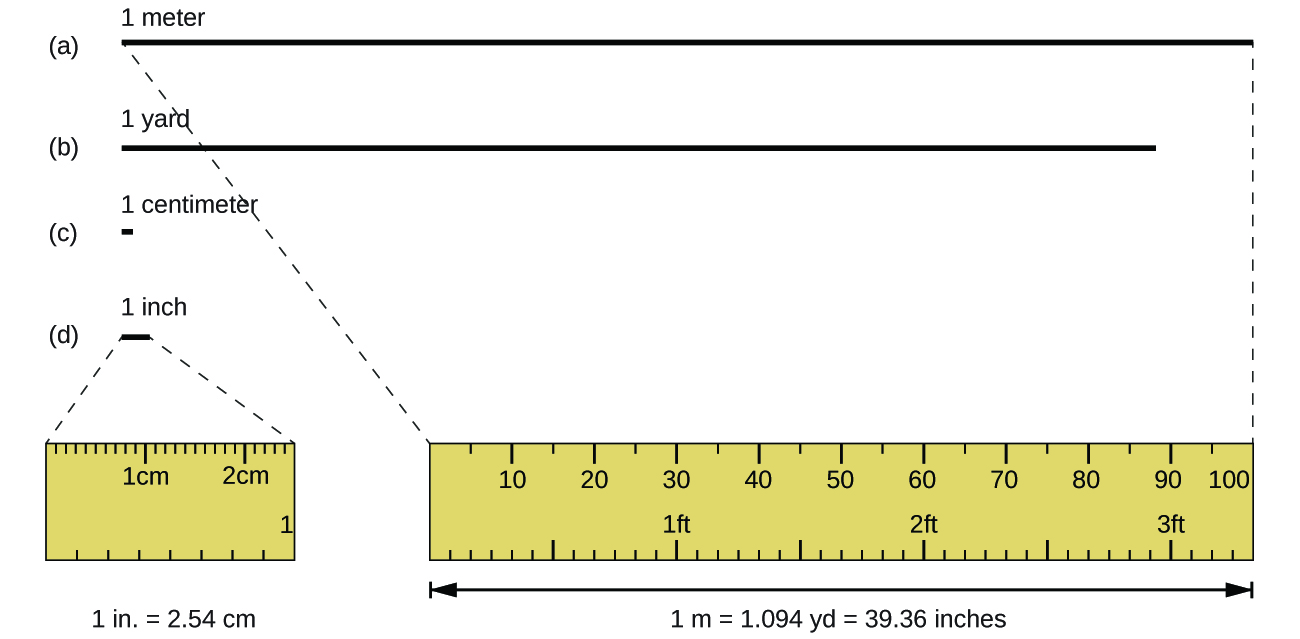
Figure \(\PageIndex{2}\): The relative lengths of 1 m, 1 yd, 1 cm, and 1 in. are shown (not actual size), as well as comparisons of 2.54 cm and 1 in., and of 1 m and 1.094 yd. (CC BY 4.0; OpenStax)
As shown in Figure \(\PageIndex{3}\), a liter is also 1,000 cm3. By definition, there are 1,000 mL in 1 L, so 1 milliliter and 1 cubic centimeter represent the same volume.
\[1\; mL = 1\; cm^3 \label{Eq2}\]
Give the abbreviation for each unit and define the abbreviation in terms of the base unit.
- kiloliter
- microsecond
- decimeter
- nanogram
- Answer a
-
The abbreviation for a kiloliter is kL. Because kilo means “1,000 ×,” 1 kL equals 1,000 L.
- Answer b
-
The abbreviation for microsecond is µs. Micro implies 1/1,000,000th of a unit, so 1 µs equals 0.000001 s.
- Answer c
-
The abbreviation for decimeter is dm. Deci means 1/10th, so 1 dm equals 0.1 m.
- Answer d
-
The abbreviation for nanogram is ng and equals 0.000000001 g.
Give the abbreviation for each unit and define the abbreviation in terms of the base unit.
- kilometer
- milligram
- nanosecond
- centiliter
- Answer a
- km (1,000 m)
- Answer b
- mg (0.001 g)
- Answer c
- ns (0.000000001 s)
- Answer d
- cL (0.01L)
Energy, another important quantity in chemistry, is the ability to perform work, such as moving a box of books from one side of a room to the other side. It has a derived unit of kg•m2/s2. (The dot between the kg and m units implies the units are multiplied together.) Because this combination is cumbersome, this collection of units is redefined as a joule (J). An older unit of energy, but likely more familiar to you, the calorie (cal), is also widely used. There are 4.184 J in 1 cal. Energy changes occur during all chemical processes and will be discussed in a later chapter.

