5.4: Alloys and Crystal Defects
- Page ID
- 444151
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Alloys
Alloys are mixtures of metals or a mixture of a metal and another element. An alloy may be a solid solution of metal elements (a homogeneous mixture) or a mixture of metallic phases (a heterogeneous mixture of two or more solutions). A solid solution can be formed by two mechanisms: (1) atom exchange or (2) interstitial mechanism. The relative size of each element in the mix plays a primary role in determining which mechanism will occur.
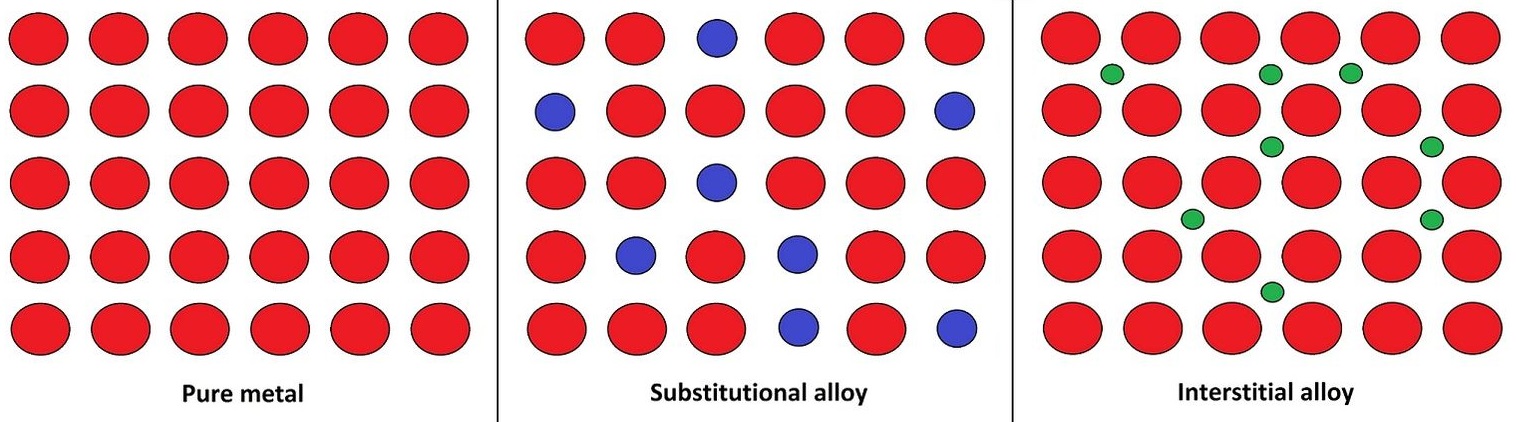 Figure \(\PageIndex{1}\): Different atomic mechanisms of alloy formation, showing pure metal, substitutional, and interstitial structures. [Wikipedia]
Figure \(\PageIndex{1}\): Different atomic mechanisms of alloy formation, showing pure metal, substitutional, and interstitial structures. [Wikipedia]Substitutional Alloys
When the atoms in a mixture are relatively similar in size, a solid solution usually occurs by the atom exchange method. In this case some of the atoms composing the metallic crystal are substituted with atoms another element. This is called a substitutional alloy. Bronze and brass are substitutional alloys in which copper atoms are substituted with tin or zinc atoms, respectively.
The bonding between two metals is best described as a combination of metallic electron "sharing" and covalent bonding, one can't occur without the other and the proportion of one to the other changes depending on the constituents involved. Metals share there electrons throughout there structure; this flow of electrons is the reason behind many of the characteristics associated with metals, including their ability to act as conductors. The different amount and strength of covalent bonds in a lattice can change depending on the specific metals involved and the ratios in which they are mixed. The covalent bonding is what is responsible for the crystal structure as well as the melting point and various other physical properties.

As the similarities between the electron structure of the metals involved in the alloy increase, the metallic character of the alloy decreases. Pure metals are useful but their applications are often limited to each individual metal's properties. Alloys allow metal mixtures that have increased resistance to oxidation, increased strength, conductivity, and melting point. Essentially any property can be manipulated by adjusting alloy concentrations. An example could be Brass Door fixtures, they are strong and resist corrosion better then pure zinc or copper, the two major metals that constitute a brass alloy. The combination also has a low melting point allowing it to be easily cast into many different shapes and sizes. There are many other aspects of substitutional alloys that could be explored in depth, but the basic concept is the idea that each individual metal in an alloy give the final product its chemical and physical properties.
Substitutional alloys played an important role in the development of human society and culture as we know it today. The Bronze age itself is named after the substitutional alloy consisting of tin in a metallic solution of copper. Ancient bronzes are very impure, or even mislabeled, containing large amounts of zinc and arsenic as well as lots of impurities. These many substitutional alloys allowed for stronger tools and weapons, they allowed for increased productivity in the workshop as well as on the battlefield. The need for raw materials like tin and copper for the production of bronze also spurred an increase in trade, since their ores are rarely found together. The current chemical understanding of substitutional alloys would not be so in depth if it weren't for the usefulness of the alloys to humans.
Interstitial Alloys
An interstitial mechanism usually occurs when the metal lattice atoms are much large than the other type of atom in the mixture. As such, the smaller atom cannot successfully replace an atom in the metal lattice. The smaller atoms become trapped in the interstices (spaces between the atoms in the metallic lattice). This is referred to as an interstitial alloy. Steel is an example of an interstitial alloy, because the very small carbon atoms fit into interstices of the iron matrix. Stainless steel is an example of a combination of interstitial and substitutional alloys, because the carbon atoms fit into the interstices, but some of the iron atoms are replaced with nickel and chromium atoms.
Crystal Defects
“Crystals are like people, it is the defects in them which tend to make them interesting!” - Colin Humphreys.
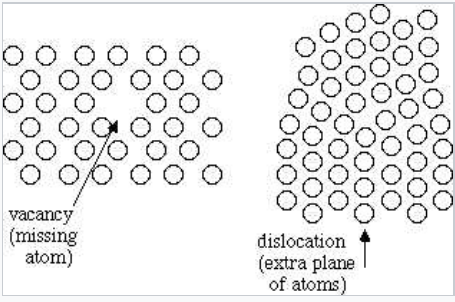
In a crystalline solid, the atoms, ions, or molecules are arranged in a definite repeating pattern, but occasional defects may occur in the pattern. Several types of defects are known, as illustrated in Figure \(\PageIndex{3}\).
Vacancies are defects that occur when positions that should contain atoms or ions are vacant. Interstitial impurities occur when atoms or ions occupy positions, called interstitial sites, located between the regular positions for atoms. Substitutional impurities occur when atoms or ions occupy regular lattice positions. In some cases, the impurity atoms may be smaller than the lattice atoms (aqua atom in Figure \(\PageIndex{3}\). When the impurity atom is too large to fit into the regular lattice positions, the substitutional impurity may distort the lattice structure (yellow atom in Figure \(\PageIndex{3}\).
Trace amounts of impurities are sometimes added to a crystal (a process known as doping) in order to create defects in the structure that yield desirable changes in its properties. For example, silicon crystals are doped with varying amounts of different elements to yield suitable electrical properties for their use in the manufacture of semiconductors and computer chips.

Because both alloys and defects can be achieved by substitutional and interstitial mechanisms, you may be wondering what the different between an alloy and a defect is. It is in part related to concentration. Generally, a defect is the result of very small concentrations of impurities, while alloys represent larger concentrations of atoms added to a structure. Alloys are usually made the intentional incorporation of atoms into a lattice. Impurities, may or may not be intentional depending on the application of the material!
Metals, by virtue of their non-directional bonding, are more energetically tolerant of defects than are covalent network or ionic solids. Because there is no strong preference for one atomic position over another, the energy of a metallic crystal is not greatly impaired by the vacancy of a single atom or by the dislocation of a group of atoms. These kinds of "mistakes" in the packing of metal atoms within crystals are collectively called defects. The deformability of metals is the direct result of defects in the crystal structure. Defects in metals such as Al and Fe are responsible for the three orders of magnitude difference between the yield stress of annealed polycrystalline samples (i.e., normal articles of commerce) and perfect single crystals.
Summary
When a molten metal is mixed with another substance, there are two mechanisms that can cause an alloy to form: (1) atom exchange or (2) interstitial mechanism. The relative size of each element in the mix plays a primary role in determining which mechanism will occur. When the atoms are relatively similar in size, the atom exchange method usually happens, where some of the atoms composing the metallic crystals are substituted with atoms of the other constituent. This is called a substitutional alloy. Examples of substitutional alloys include bronze and brass, in which some of the copper atoms are substituted with either tin or zinc atoms. Alloys can be also formed by inserting smaller atoms into holes in the metal lattice (interstitial alloys). Although the elemental composition of most alloys can vary over wide ranges, certain metals combine in only fixed proportions to form intermetallic compounds.
References
- Smallman, R. E., Ngan, A. H. W., & Smallman, R. E. (2007). Physical metallurgy and advanced materials. Amsterdam: Butterworth Heinemann.
- Wang, F. E.. (2005). Bonding theory for metals and alloys. Amsterdam: Elsevier.
- Dickinson, O. T. P. K. (1994). The Aegean Bronze age. Cambridge world archeology. Cambridge: Cambridge University Press.

