2.3: Acid-Base Theories and Concepts
- Page ID
- 443614
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)In a very real sense, we can make an acid anything we wish. The differences between the various acid-base concepts are not concerned with which is 'right', but which is most convenient to use in a particular situation.
James E. Huheey, Ellen A. Keiter, and Richard L. Keiter
There are three primary theories of acid-base chemistry that are often taught together: Arrhenius theory, Brønsted-Lowry theory, and Lewis acid-base theory. Each theory is introduced below.
Arrhenius Acids and Bases
To qualify as an Arrhenius acid, upon the introduction to water, the chemical must either cause, directly or otherwise:
- an increase in the aqueous hydronium concentration, or
- a decrease in the aqueous hydroxide concentration.
The Swedish chemist Svante Arrhenius attributed the properties of acidity to hydrogen ions (\(\ce{H^{+}}\)) in 1884. An Arrhenius acid is a substance that, when added to water, increases the concentration of \(\ce{H^{+}}\) ions in the water. Note that chemists often write \(\ce{H^{+}(aq)}\) and refer to the hydrogen ion when describing acid-base reactions, but the free hydrogen nucleus does not exist alone in water. It exists in a hydrated form which for simplicity is often written as the hydronium (hydroxonium) ion, \(\ce{H3O^{+}}\). Thus, an Arrhenius acid can also be described as a substance that increases the concentration of hydronium ions when added to water. This definition stems from the equilibrium dissociation (self-ionization) of water into hydronium and hydroxide (\(\ce{OH^{-}}\)) ions:
\[\ce{H_2O(l) + H_2O(l) ⇌ H_3O^{+}(aq) + OH^{-}(aq)} \nonumber\]
with \(K_w\) defined as \(\ce{[H^{+}][OH^{-}]}\).
The value of \(K_w\) varies with temperature, as shown in the table below where at 25 °C \(K_w\) is approximately \(1.0 \times 10^{-14}\), i.e. \(pK_w= 14\).
| Water temperature | Kw / 10-14 | pKw |
|---|---|---|
| 0 °C | 0.112 | 14.95 |
| 25 °C | 1.023 | 13.99 |
| 50 °C | 5.495 | 13.26 |
| 75 °C | 19.95 | 12.70 |
| 100 °C | 56.23 | 12.25 |
In pure water the majority of molecules are \(\ce{H2O}\), but the molecules are constantly dissociating and re-associating, and at any time a small number of the molecules (about 1 in 107) are hydronium and an equal number are hydroxide. Because the numbers are equal, pure water is neutral (not acidic or basic) and has an electrical conductivity of 5.5 microSiemen, μS/m. For comparison, sea water's conductivity is about one million times higher, 5 S/m (due to the dissolved salt).
Although the term proton is often used for \(\ce{H^{+}}\), this should really be reserved for \(\ce{H}\) (protium) not \(\ce{D}\) (deuterium) or \(\ce{T}\) (tritium). The more general term, hydron covers all isotopes of hydrogen.
To qualify as an Arrhenius base, upon the introduction to water, the chemical must either cause, directly or otherwise:
- a decrease in the aqueous hydronium concentration, or
- an increase in the aqueous hydroxide concentration.
The definition is expressed in terms of an equilibrium expression:
\[\text{acid} + \text{base} ⇌ \text{conjugate base} + \text{conjugate acid}. \nonumber\]
With an acid, \(\ce{HA}\), the equation can be written symbolically as:
\[\ce{HA + B ⇌ A^{-} + HB^{+}} \nonumber\]
The double harpoons sign, \(\ce{<=>}\), is used because the reaction can occur in both forward and backward directions. The acid, \(\ce{HA}\), can lose a hydron to become its conjugate base, \(\ce{A^{-}}\). The base, \(\ce{B}\), can accept a hydron to become its conjugate acid, \(\ce{HB^{+}}\). Most acid-base reactions are fast so that the components of the reaction are usually in dynamic equilibrium with each other.
Brønsted-Lowry Acids and Bases
While the Arrhenius concept is useful for describing many reactions, it has limitations. In 1923, chemists Johannes Nicolaus Brønsted and Thomas Martin Lowry independently recognized that acid-base reactions involve the transfer of a hydron. A Brønsted-Lowry acid (or simply Brønsted acid) is a species that donates a hydron to a Brønsted-Lowry base. The Brønsted-Lowry acid-base theory has several advantages over the Arrhenius theory. Consider the following reactions of acetic acid (\(\ce{CH3COOH}\)):
\[\ce{CH_3COOH + H_2O ⇌ CH_3COO^{-} + H_3O^{+}} \nonumber\]
\[\ce{CH_3COOH + NH_3 ⇌ CH_3COO^{-} + NH_4^{+}} \nonumber\]
Both theories easily describe the first reaction: \(\ce{CH3COOH}\) acts as an Arrhenius acid because it acts as a source of \(\ce{H3O^{+}}\) when dissolved in water, and it acts as a Brønsted acid by donating a hydron to water. In the second example \(\ce{CH3COOH}\) undergoes the same transformation, in this case donating a hydron to ammonia (\(\ce{NH3}\)), but it cannot be described using the Arrhenius definition of an acid because the reaction does not produce hydronium ions.
To qualify as an Brønsted-Lowry acid, the chemical must either cause, directly or otherwise:
- donate a proton.
Conversely, to qualify as an Brønsted-Lowry base, the chemical must either cause, directly or otherwise:
- accept a proton.
Lewis Acids and Bases
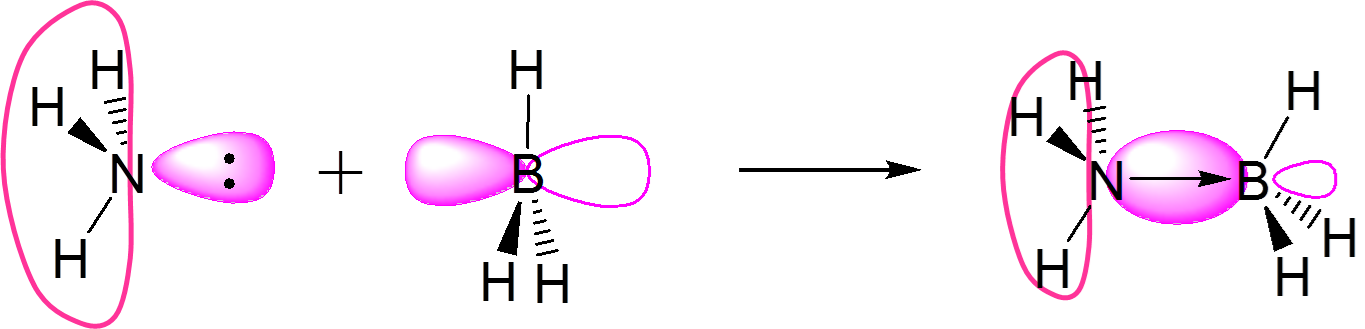
A third concept was proposed in 1923 by Gilbert N. Lewis which includes reactions with acid-base characteristics that do not involve a hydron transfer. A Lewis acid is a species that reacts with a Lewis base to form a Lewis adduct. The Lewis acid accepts a pair of electrons from another species; in other words, it is an electron pair acceptor. Brønsted acid-base reactions involve hydron transfer reactions while Lewis acid-base reactions involve electron pair transfers. All Brønsted acids are Lewis acids, but not all Lewis acids are Brønsted acids.
\[\ce{BF3 + F^{-} <=> BF4^{-}} \nonumber\]
\[\ce{NH3 + H^{+} <=> NH4^{+}} \nonumber\]
In the first example \(\ce{BF3}\) is a Lewis acid since it accepts an electron pair from the fluoride ion. This reaction cannot be described in terms of the Brønsted theory because there is no hydron transfer. The second reaction can be described using either theory. A hydron is transferred from an unspecified Brønsted acid to ammonia, a Brønsted base; alternatively, ammonia acts as a Lewis base and transfers a lone pair of electrons to form a bond with a hydrogen ion.
To qualify as an Lewis acid, the chemical must:
- accept an electron pair.
Conversely, to qualify as an Lewis base, the chemical must:
- donate an electron pair.The concept of acids and bases is often associated with the movement of hydrogen ions from one molecule or ion to another. However, a host of acid-base concepts have been developed to help chemists organize and make sense of a wide range of reactions (Table 6.1).
Other Definitions
Lux-Flood
This acid-base theory was a revival of the oxygen theory of acids and bases, proposed by German chemist Hermann Lux in 1939 and further improved by Håkon Flood circa 1947. It is still used in modern geochemistry and for the electrochemistry of molten salts. This definition describes an acid as an oxide ion (\(\ce{O^{2-}}\)) acceptor and a base as an oxide ion donor. For example:
\[\ce{MgO (base) + CO2 (acid) <=> MgCO3} \nonumber\]
\[\ce{CaO (base) + SiO2 (acid) <=> CaSiO3}\nonumber\]
\[\ce{NO3^{-} (base) + S2O7^{2-} (acid) <=> NO2+ + 2 SO4^{2-}}\nonumber\]
Usanovich
Mikhail Usanovich developed a general theory that does not restrict acidity to hydrogen-containing compounds, and his approach, published in 1938, was even more general than the Lewis theory. Usanovich's theory can be summarized as defining an acid as anything that accepts negative species, anions or electrons or donates positive ones, cations, and a base as the reverse. This definition could even be applied to the concept of redox reactions (oxidation-reduction) as a special case of acid-base reactions. Some examples of Usanovich acid-base reactions include:
- species exchanged: anion \(\ce{O^{2-}}\) \[\ce{Na2O (base) + SO3 (acid) → 2Na^{+} + SO4^{2-}} \nonumber\]
- species exchanged: anion \(\ce{S^{2-}}\) \[\ce{3(NH4)2S (base) + Sb2S5 (acid) → 6NH4+ + 2SbS4^{3-}} \nonumber\]
- species exchanged: electron \[\ce{2Na (base) + Cl2 (acid) → 2Na^{+} + 2Cl^{-} } \nonumber\]
A comparison of the above definitions of Acids and Bases shows that the Usanovich concept encompasses all of the others but some feel that because of this it is too general to be useful.
A Summary of Acid-Base Concepts
Some concepts involve defining acids and bases in particular ways that allow for the understanding of particular types of chemical systems. For example, the familiar Arrhenius and Brønsted acid and base concepts used in general chemistry help chemists make sense of the behavior of compounds which can transfer H+ ions among themselves, often in aqueous solution. However, the solvent system acid-base concept defines acids and bases in terms of the transfer of a lone-pair bearing group and is particularly useful for conceptualizing the reactivity of main group halides, oxides, and related compounds. Some acid-base definitions seek to encompass an extremely wide range of chemical reactions. For instance, the Lewis acid-base definition encompasses the Arrhenius , Brønsted, and solvent system definitions and has also found wide use in inorganic chemistry owing to the ease with which Lewis acid-base interactions may be described by the Frontier Orbital approach in terms of interacting molecular orbitals on the acid and base.
| Definition | Theoretical paradigm and notable features. | Acid | Base | Illustrative sample reactions |
|---|---|---|---|---|
|
Arrhenius (1894) |
Interested in what the substance does to the state of an aqueous solution. In particular it assesses proton donation to & removal from water using [H3O+] as a proxy. Readily accommodates the pH concept as a measure of the state of a solution. |
Increases [H3O+] |
Decreases [H3O+] |
\(\underset{acid}{HCl}~+~H_2O~\rightarrow~H_3O^+~+~Cl^-\) \(\underset{base}{NH_3}~+~H_2O~\rightarrow~NH_4^+~+~OH^-\) |
|
Brønsted-Lowry (1923) |
Envisions acid-base reactivity in terms of the transfer of an H+ from one substance to another. Allows for conjugate acids and bases and solvent autoionization. | Donates H+ | Accepts H+ |
\(\underset{acid}{HCl}~+~\underset{base}{NH_3}~\rightarrow~\underset{conj.~acid}{NH_4^+}~+~\underset{conj.~base}{Cl^-}\) \(\underset{acid}{HOAc}~+~\underset{base}{NH_3}~\rightarrow~\underset{conj.~acid}{NH_4^+}~+~\underset{conj.~base}{OAc^-}\) \(\underset{amphoteric}{2~H_2O}~\rightarrow~\underset{conj.~acid}{H_3O^+}~+~\underset{conj.~base}{OH^-}\) |
|
Lux-Flood (1939-~47) |
Describes reactions involving oxides and oxyanions in terms of the transfer of oxide ion (O2-). Mainly used in geochemistry, although it also can be used to describe some redox reactions. | Oxide acceptor | Oxide donor |
\(\underset{acid}{SiO_2}~+~\underset{base}{CaO}~\rightarrow~CaSiO_3\) \(\underset{base}{H_2O}~+~\underset{acid}{CO}~\rightarrow~H_2~+~CO_2\) |
| Solvent System | Applies aspects of the Arrhenius , Brønsted-Lowry, and Lux-Flood acid base concepts to solvent cation & anion formation in a generalized reaction. Can be used to describe solution chemistry in nonaqueous solvent systems like BrF3. | Is a solvent cation or increases the solvent cation concentration, often by receiving a lone pair- bearing group | Is a solvent anion or increases the solvent anion concentration, often by donating a lone pair- bearing group |
\(\underset{acid}{SbF_5}~+~\underset{base}{BrF_3}~\rightarrow~\underset{conj.~base}{SbF_6^-}~+~\underset{conj.~acid}{BrF_2^+}\) \(\underset{amphoteric}{2~BrF_3}~\rightarrow~\underset{conj.~acid}{BrF_2^+}~+~\underset{conj.~base}{BrF_4^-}\) |
| Lewis (1923) | Envisions acid-base reactivity in terms of electron pair donation. Encompasses the Arrhenius , Brønsted-Lowry, Lux-Flood, and Solvent System definitions and readily integrates with molecular orbital descriptions of chemical reactivity in Frontier orbital theory. | Accepts an electron pair | Donates an electron pair | \(\underset{base}{:NH_3}~+~\underset{acid}{BF_3}~\rightarrow~H_3N~+~BF_3\) |
| Nucleophile-Electrophile | Applies the Lewis concept to organic reactivity. Nucleophiles are Lewis bases which tend to react to form a bond with Lewis acid sites called electrophilic centers. |
(The electrophile) |
(The nucleophile) Donates an electron pair to form a bond to an electrophile |
\(\underset{base}{Br^-}~+~\underset{acid}{CH_3-Cl}~\rightarrow~Br-CH_3~+~Cl^-\) |
|
Usanovich (1939) |
Extends Lewis theory to include the donation and acceptance of any number of electrons, whether through the formation of an adduct or electron transfer. | Accepts electrons | Donates electrons |
\(\underset{base}{:NH_3}~+~\underset{acid}{BH_3}~\rightarrow~\underset{adduct}{H_3N-BH_3}\) \(\underset{acid}{Fe^{2+}}~+~\underset{base}{Zn^0}~\rightarrow~Fe^0~+~Zn^{2+}\) |
| Frontier Orbital (1960s) | Envisions Lewis Acid-base/Electrophile-nucleophile reactions in terms of the donation and acceptance of electrons between the reactant's frontier orbitals. Specifically, the reaction is envisioned in terms of donation of the base's HOMO electrons into the acid's LUMO level. | Possesses a LUMO capable of forming an occupied bonding MO on mixing with a base's HOMO. | Possesses an electron-bearing HOMO capable of forming a filled bonding MO on mixing with an acid's LUMO. |
base acid adduct |
References
Huheey, J. E.; Keiter, E. A.; Keiter, R. L., Inorganic Chemistry: Principles of Structure and Reactivity. 4th ed.; HarperCollins: New York, NY, 1993, pg. 318.