1.7: How to Write a Ground State Electron Configuration
- Page ID
- 443910
Estimated Time to Read: 5 min
Basic Steps
Electron configurations list every subshell for an atom or ion and how many electrons are in each subshell. Subshells are described by writing the principal quantum number n followed by the symbol for the angular momentum quantum number l (s, p, d, or f). The the total number of electrons in each subshell is written as a superscript. The lowest energy subshell is written first, and additional subshells are written in order of increasing energy.
Write the ground state electron configuration for sodium, Na.
Solution: Method 1
- Find the number of electrons in the atom: Sodium has 11 electrons.
- List the subshells following the model shown in Figure 1.6.3: 1s 2s 2p 3s
- Fill the subshells until all electrons have been accounted for. Remember: each s subshell can hold 2 electrons, each p subshell can hold six electrons, each d subshell can hold 10 electrons, and each f subshell can hold 14 electrons: 1s2 2s2 2p6 3s1
Solution: Method 2
- Locate the atom on the periodic table.
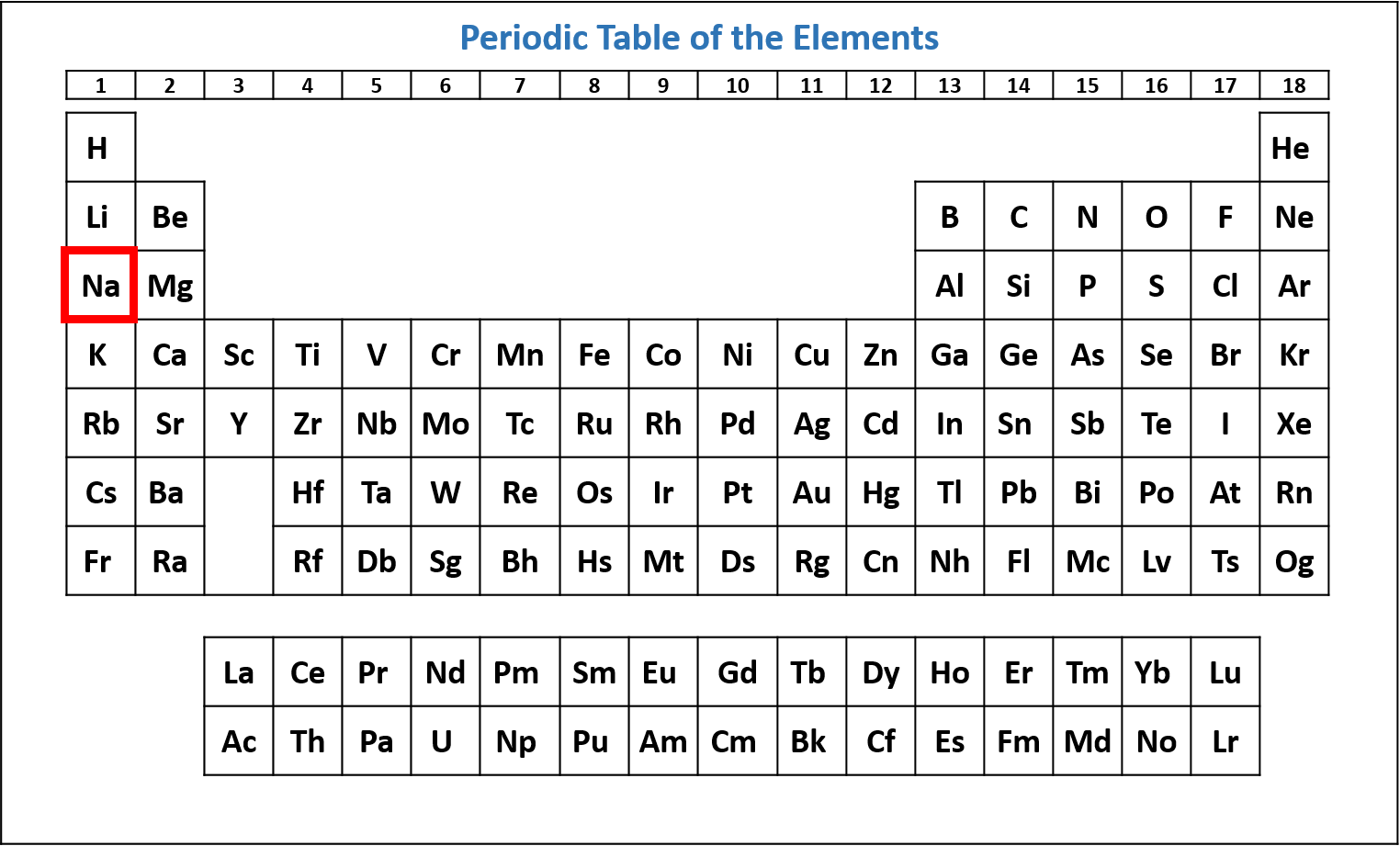
Figure \(\PageIndex{1}\): Periodic table of the elements with the location of sodium (Na) highlighted. (CC-BY-NC-SA; Kathryn A. Newton) - Starting at hydrogen and the 1s subshell, read across each row of the periodic table until you get to your chosen element. As you read across each row, list each subshell and the number of electrons in the subshell.
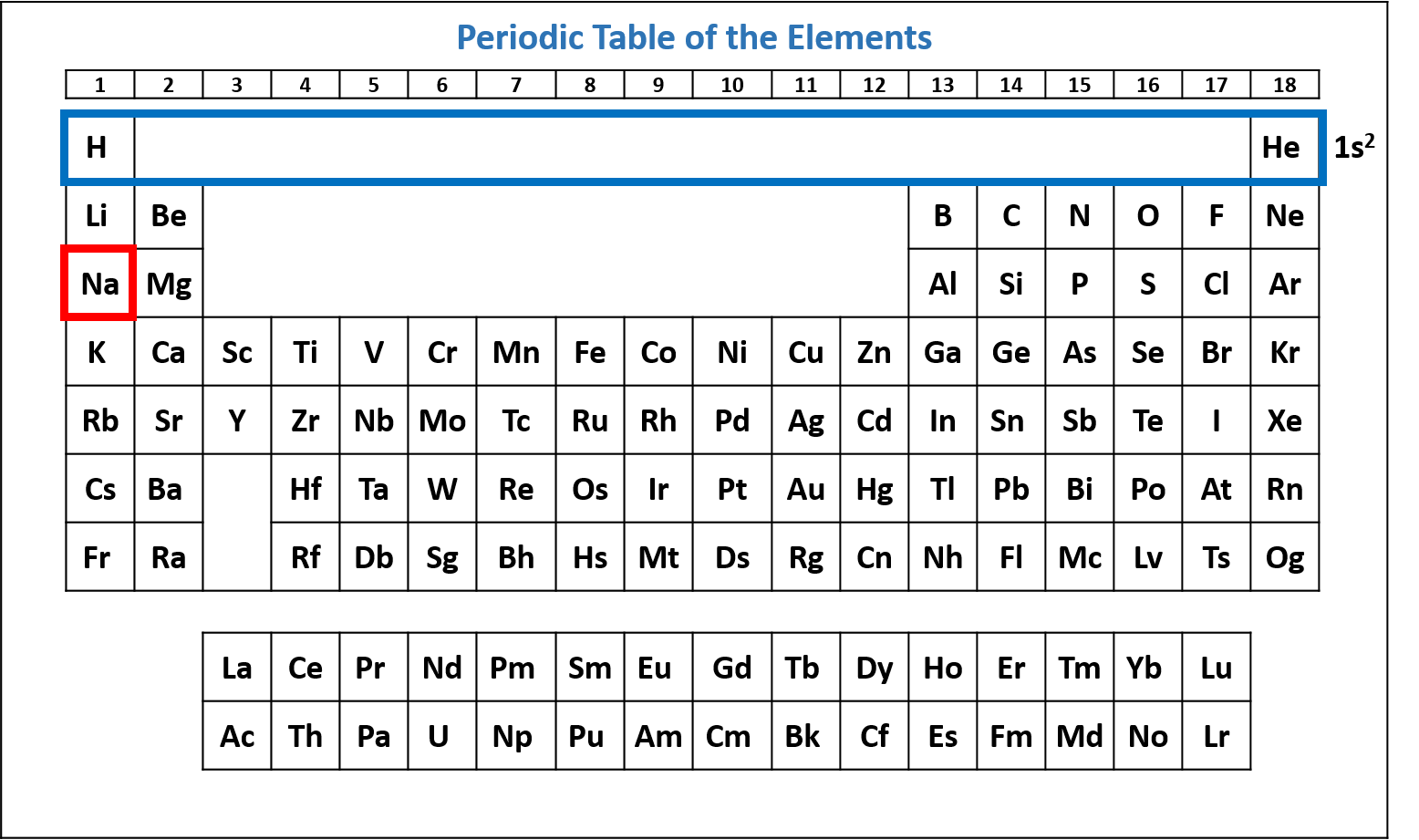
Figure \(\PageIndex{2}\): Periodic table of the elements with the 1s2 subshell highlighted. Na has two electrons in the 1s subshell. (CC-BY-NC-SA; Kathryn A. Newton) 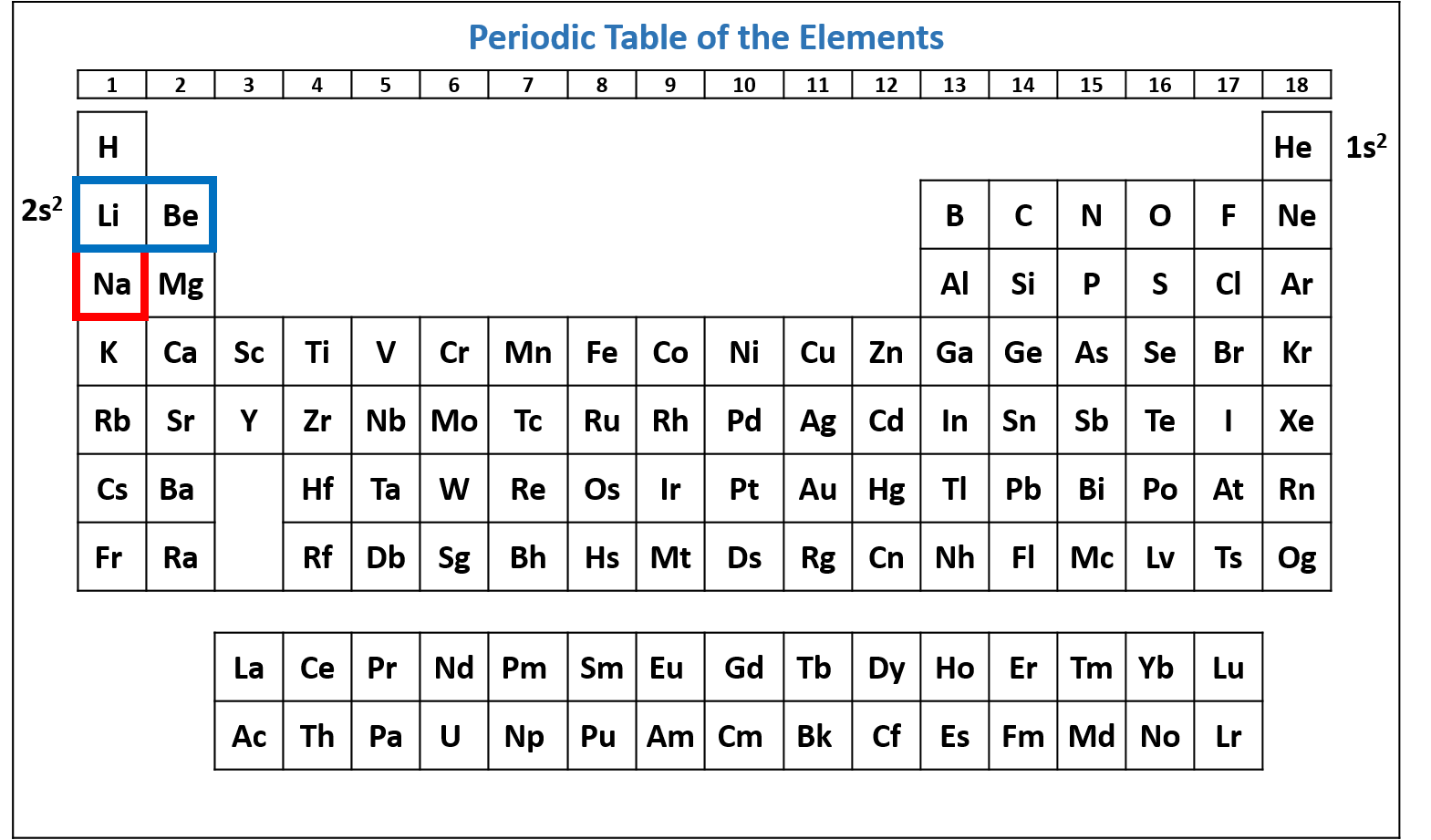
Figure \(\PageIndex{3}\): Periodic table of the elements with the 2s2 subshell highlighted. Na has two electrons in the 2s subshell. (CC-BY-NC-SA; Kathryn A. Newton) 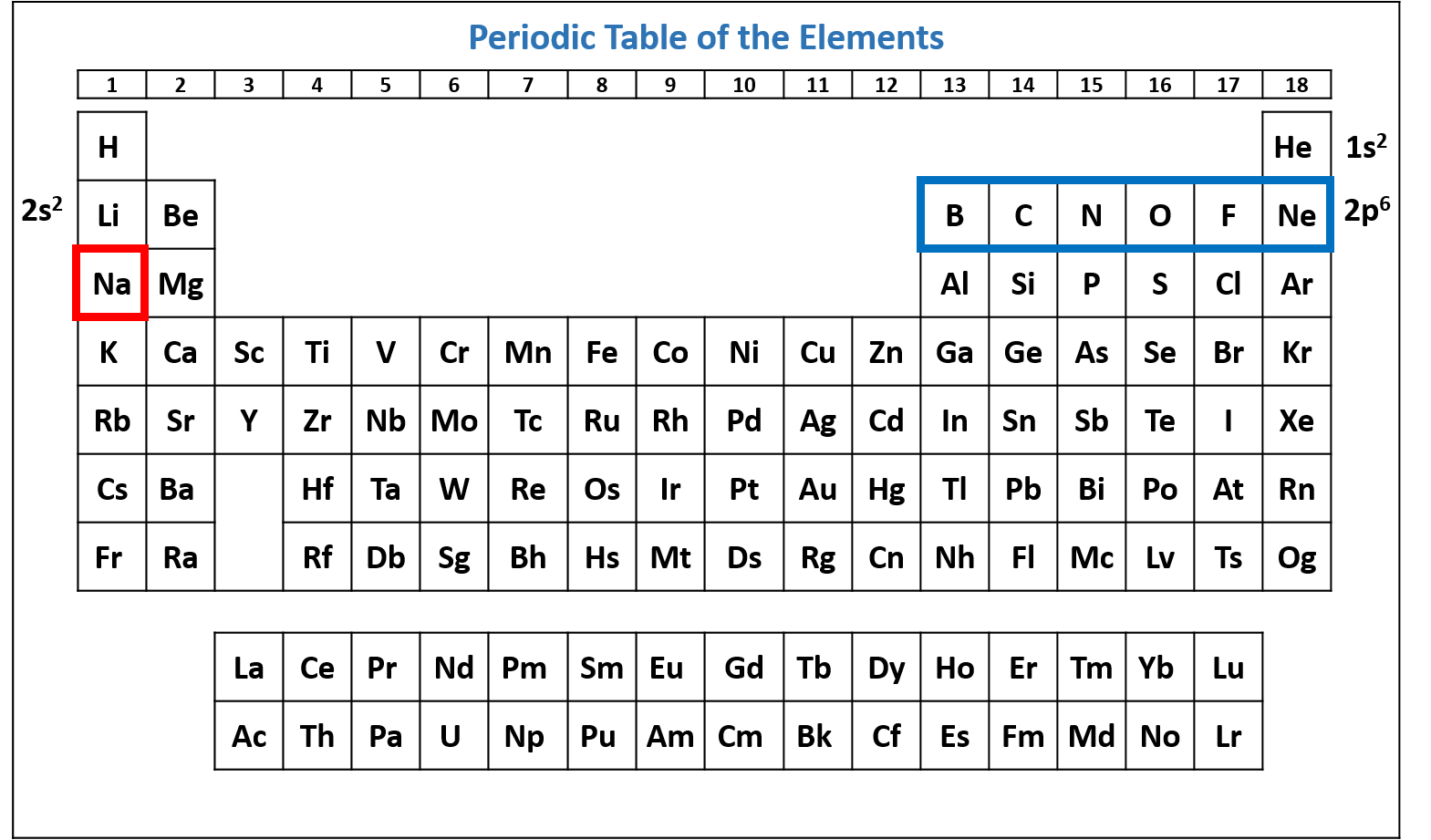
Figure \(\PageIndex{4}\): Periodic table of the elements with the 2p6 subshell highlighted. Na has six electrons in the 2p subshell. (CC-BY-NC-SA; Kathryn A. Newton) 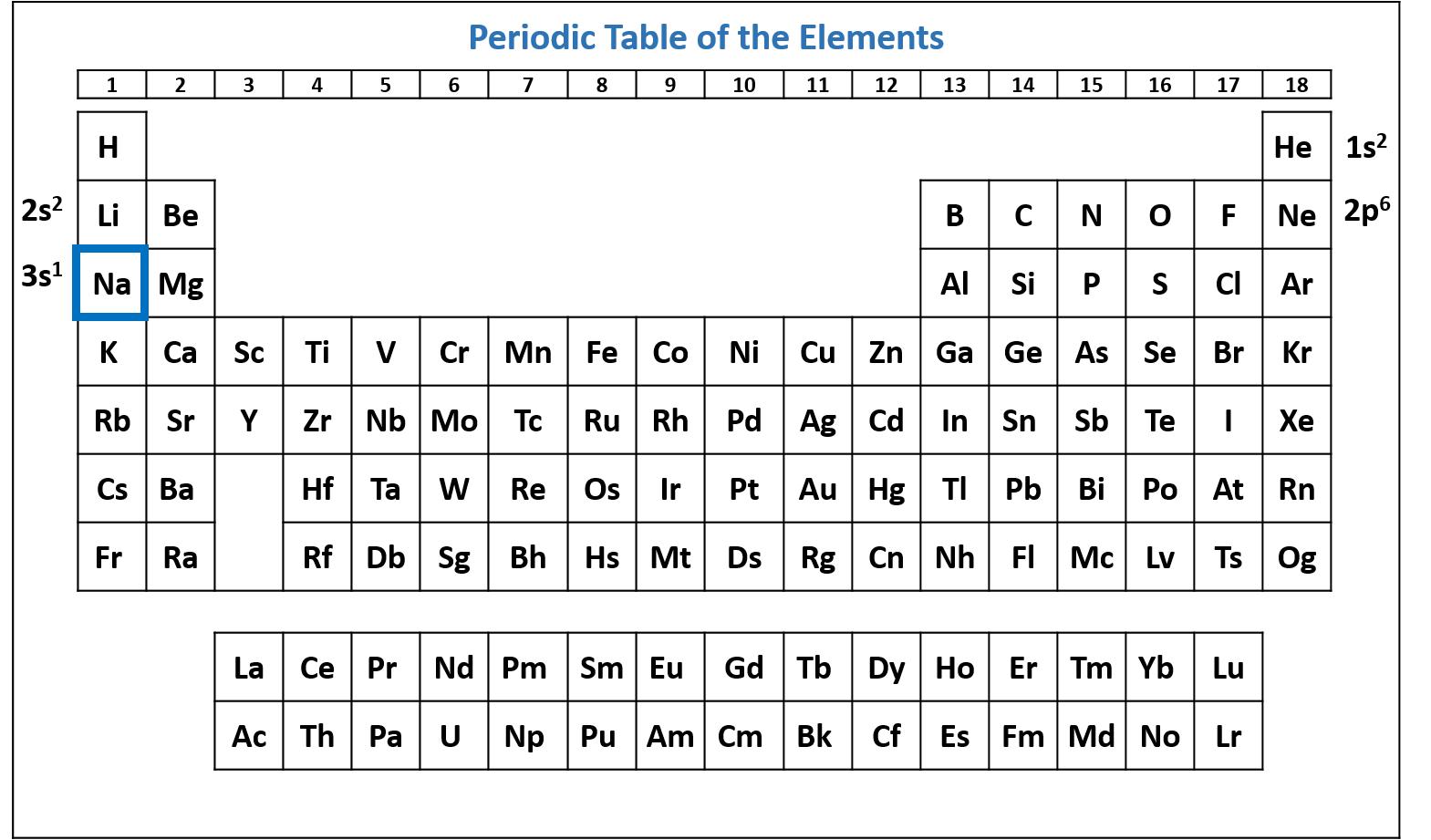
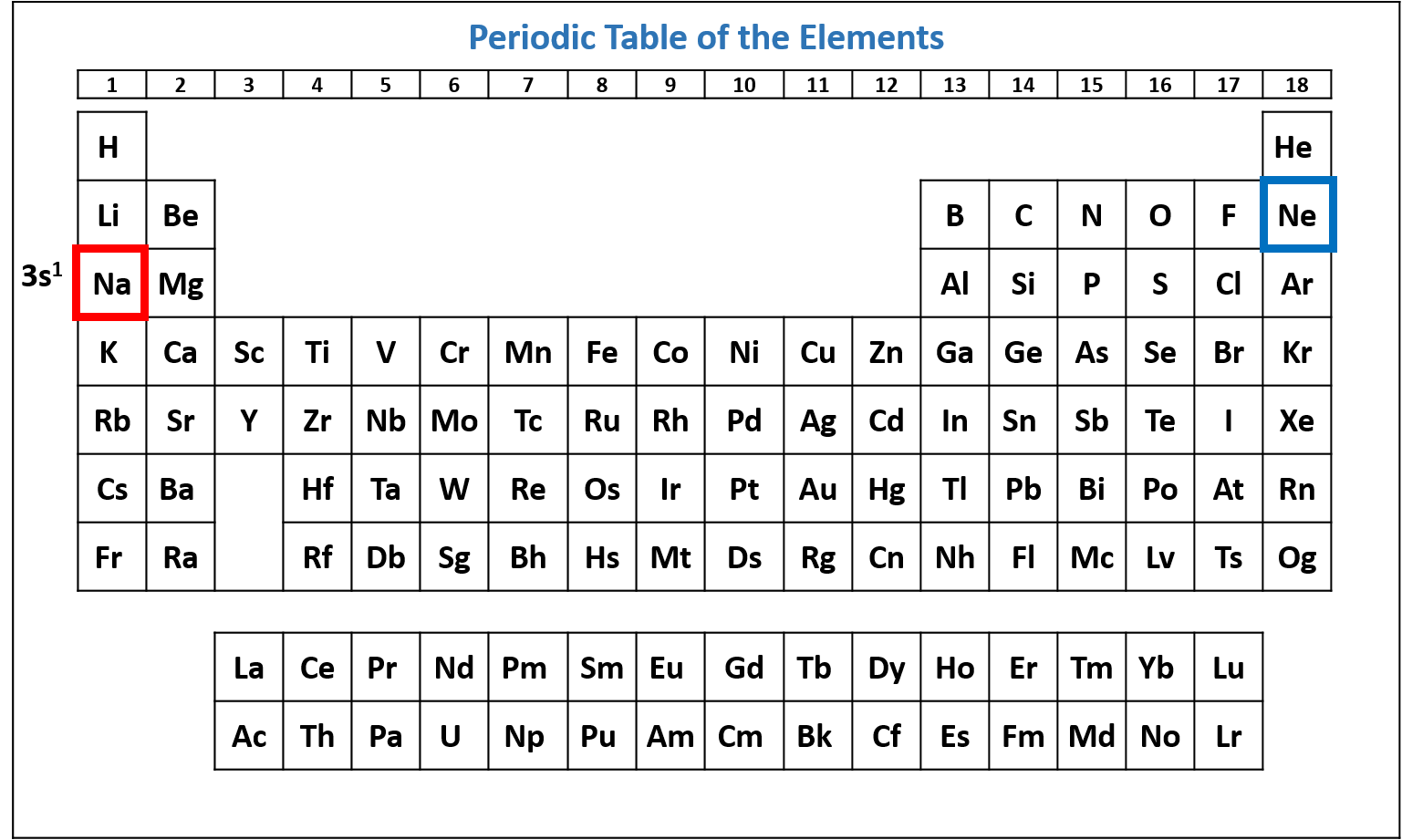
Figure \(\PageIndex{5}\): Periodic table of the elements with the 3s1 subshell highlighted. Na has one electron in the 3s subshell. (CC-BY-NC-SA; Kathryn A. Newton) - Combine the list into a single electron configuration. Na's electron configuration is 1s2 2s2 2p6 3s1.
Noble Gas Configurations
Because writing the entire electron configuration can become cumbersome, there is a shorthand option. It is done by using the symbol of the noble gas in the period above the element to represent the electron configuration before it.
Write the noble gas electron configuration for sodium (Na).
Solution
1. Locate the atom on the periodic table.
2. Locate the noble gas element in the period above the element of interest.
3. Continue the electron configuration from the noble gas until you reach the element of interest.
4. Put the noble gas in brackets and write the remainder of the electron configuration.
Na has the same electron configuration as Ne with the addition of 3s1.
Na's noble gas configuration is [Ne]3s1.
Ion Configurations
Cations
Cations are formed when a neutral atom loses electrons. To write an electron configuration for a cation, start by writing the electron configuration for the neutral atom. Then determine the number of electrons that were lost to form the cation. (The loss of a single electron results in a +1 charge, the loss of two electrons results in a +2 charge, and so on.) Subtract the number of lost electrons from the highest energy shell in the neutral configuration. When there is more than one orbital with the same principal quantum number, the electrons will be removed from the d subshell before any are removed from the p subshell and will be removed from the p subshell before any are removed from s subshell.
Write the electron configuration for Si4+.
Solution
1. Write the configuration of the neutral atom. Si: 1s2 2s2 2p6 3s2 3p2
2. Determine how many electrons were lost. Si4+ was formed by the loss of four electrons.
3. Remove electrons from the highest shell, starting with the highest energy subshell.
Si+: 1s2 2s2 2p6 3s2 3p1
Si2+: 1s2 2s2 2p6 3s2 3p0
Si3+: 1s2 2s2 2p6 3s1
Si4+: 1s2 2s2 2p6 3s0, which is typically written as 1s2 2s2 2p6 or as [Ne]
Anions
Anions are formed when a neutral atom gains electrons. To write the electron configuraiton for a cation, start by writing the electron configuration for the neutral atom. Then determine the number of electrons that were added to form the anion. (The addition of a single electron results in a -1 charge, the addition of two electrons results in a -2 charge, and so on.) Add the additional electrons to the highest energy shell in the neutral configuration. When there is more than one orbital with the same principal quantum number, the electrons will be added to any partially filled subshells first, and any remaining electrons will be added to the s subshell before the p subshell before the d subshell.
Write the electron configuration for As3-.
Solution
1. Write the configuration of the neutral atom. As: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3
2. Determine how many electrons were gained. As3- was formed by the addition of three electrons.
3. Add electrons from the highest shell, starting with any partially filled subshells.
As-: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p4
As2-: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5
As3-: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 or [Kr]
Problems
Write the expanded and shortened ground state electron configuration for Cl.
- Answer
-
Expanded: 1s2 2s2 2p6 3s2 3p5
Noble Gas: [Ne] 3s2 3p5
Write the ground state electron configuration for P3-.
- Answer
-
Expanded: 1s2 2s2 2p6 3s2 3p3
Noble Gas: [Ar]
References
- Housecroft, Catherine E. and Alan G. Sharpe, “Inorganic Chemistry,” 3rd ed. England: Pearson Education Limited: 2008.
- Tro, Nivaldo J. “Chemistry: A Molecular Approach,” 8th ed. Upper Saddle River, New Jersey: Prentice Hall: 2007.
- Silberberg, Martin S. "Chemistry: The Molecular Nature of Matter and Change," 4th ed. Boston: McGraw Hill: 2006.

