1-1 Identify the number of valence electrons for each of the following elements. Then, identify the maximum number of covalent bonds it can form with other atoms while keeping a neutral net charge.
a) Oxygen
b) Carbon
c) Chlorine
d) Sulfur
e) Hydrogen
f) Boron
1-2 Which of the following atoms can bond with Br - to satisfy the octet rule?
a) Mg+2
b) O-2
c) Cl-
d) K+
1-3 Draw the Lewis dot structure of the correct answer from the previous problem 1-2 (a) - (d).
1-4 Identify which of the following compounds could not form due to an unfilled octet.
a) NCl3
b) NaOH
c) PCl
d) CF4
Lewis Structures
1-5 Draw the Lewis structures for the following compounds.
a) H2O
b) O3
c) BH3
d) SOCl2
1-6 Name the element that corresponds to each electronic configuration and identify how many valence electrons it has.
a) 1s22s22p6
b) 1s22s22p63s2
c) 1s22s22p4
d) 1s22s22p63s23p64s23d104p5
1-7 Draw the Lewis structures for PF3 and PF5.
1-8 Draw the Lewis structure for furan.

1-9 Identify the correct Lewis structure for hydroperoxyl, HO2.
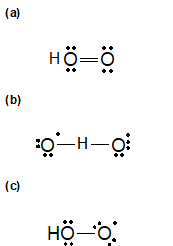
Electronegativity and Bond Polarity
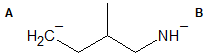
1-10 For the indicated bond in each of the following compounds, identify which atom is more electronegative, if applicable.
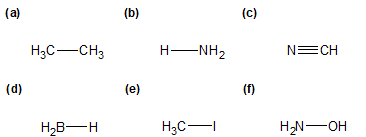
1-11 For each of the compounds in the previous problem, add a dipole moment arrow.
1-12 For the following compounds, draw the structural formula. Then calculate the formal charge on each atom other than hydrogen.
a) N(CH3)4+
b) HSO4-
c) CH3CC-
1-13 Identify the formal charge for the following compounds.
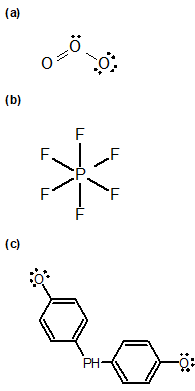
1-14 Identify the formal charges for the central carbon in each of the following compounds.
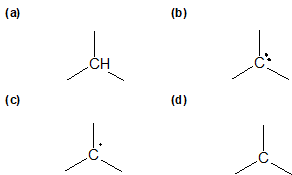
Ionic Structures
1-15 Identify the substituent ions that make up the following salts.
a) NaCl
b) MgBr2
c) KNO3
d) NaH2PO4
1-16 Identify the products of the following reactions.

1-17 Give the correct nomenclature or write the correct chemical formula for the following ionic compounds.
a) NaCN
b) calcium oxalate
c) Al(OH)3
d) tin (II) phosphate
e) potassium hypochlorite
Resonance
1-18 For the following structure, draw its resonance structure(s).
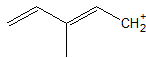
1-19 Which resonance form from the previous problem has the most stable carbocation? Explain your answer.
1-20 Draw the important resonance forms to show the delocalization of charges in the following compounds.
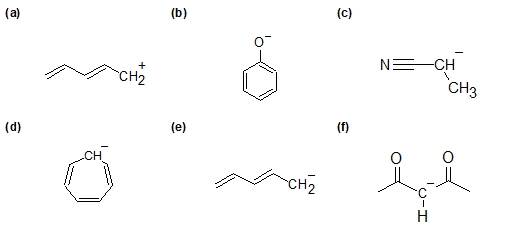
1-21 Explain how resonance contributes to the lower pKa of acetic acid CH3CO2H (pKa= 4.75) compared to the pKa of ethanol CH3CH2OH (pKa=15.9).
1-22 Draw the resonance structure(s) for fulminic acid (HCNO).
1-23 Identify the molecular and empirical formula for the following structures.
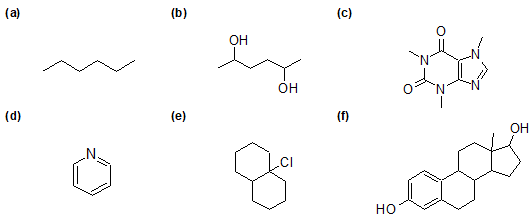
1-24 Draw all possible structural formulas for the following compounds.
a) C4H10
b) CHN
c) C4H9Cl
1-25 True or False: You can always calculate the exact molecular weight of a molecule from its empirical formula.
1-26 For the following molecular formulas, provide the empirical formula.
a) C4H4O2
b) C8H6N2
c) C9H21N3O3
Acids and Bases - Arrhenius, Bronsted-Lowry, and Lewis
1-27 Briefly explain the three different definitions of acids and bases.
1-28 Calculate the Ka of nitric acid (HNO3). pKa of nitric acid is -1.4.
1-29 Rank the following in order of decreasing acidity: NH4+ HF H3O+ H2O
1-30 Rank the following in order of decreasing basicity: HSO4- H2O CH3COO- NH2-
1-31 Identify which compound is the stronger base. Identify which compound is the stronger acid.
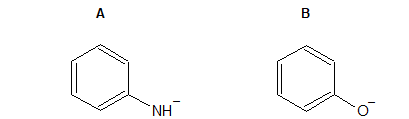
1.32 Identify which group is more likely to grab a H+.