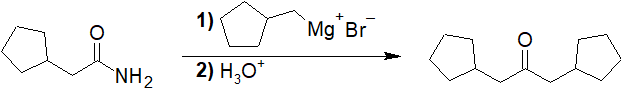General Review
22-1 Suggest a carboxylic acid and an acid derivative that could be reacted together to form the following molecule.

22-2 For the following reaction, predict the product if:
- only one equivalent of the Grignard reagent was used (and the product could be isolated)
- if two equivalents were used (followed by an acid workup)
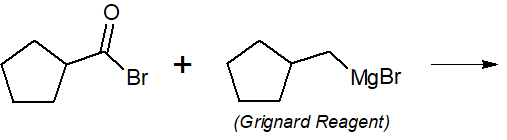
22-3 Provide the final products of the following reactions.
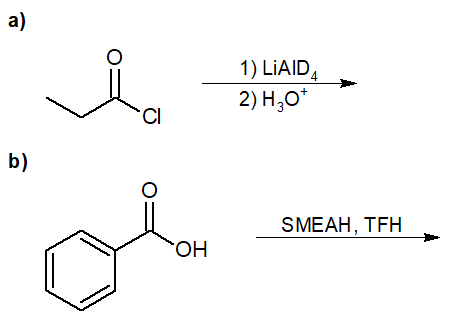
22-4 Predict the final product of the following reaction.
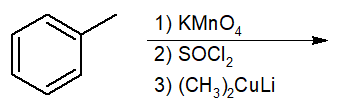
Interconversion of Acid Derivatives by Nucleophilic Acyl Substitution
22-5 Predict the interconverted acid derivatives of the following reactions.
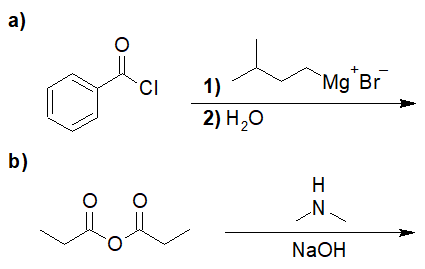
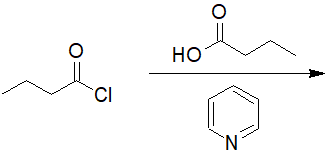
22-6 Predict the structure of the product and give its IUPAC nomenclature.
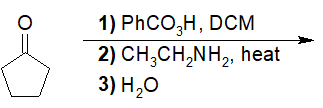
22-7 Choose the correct answer for the product of the following reaction.
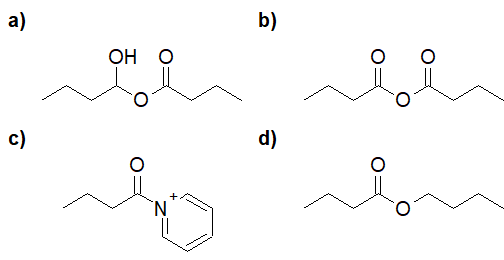
Transesterification
22-8 Choose the correct IUPAC nomenclature of the product of the following reaction.
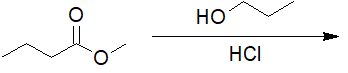
a) ethyl butanoate
b) propan-2-yl butanoate
c) dipropyl carbonate
d) propyl butanoate
22-9 Explain why transesterification can be done under acidic or basic conditions.
22-10 Give the products of the following transesterification reactions.

Hydrolysis of Carboxylic Acid Derivatives
22-11 Provide the correct structure of the product of the following hydrolysis reaction.
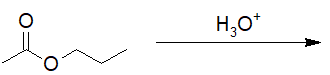
22-12 Provide the structure of all the products resulting from the hydrolysis of the following triglyceride.
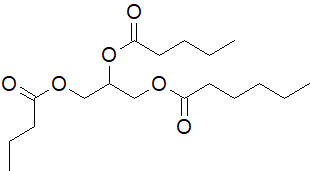
22-13 Provide the structures and IUPAC nomenclature of the products.
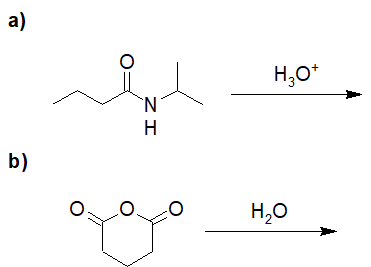
Reduction of Acid Derivatives
22-14 Give the structure of the product of the following reaction.
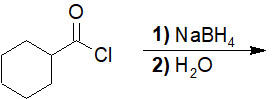
22-15 Provide the structure of all the products (including leaving groups) formed in the following reaction.
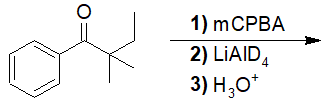
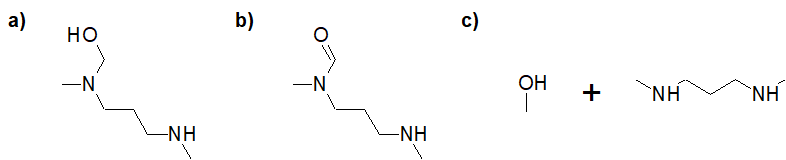
22-16 Choose the correct answer that gives the products of a fully reduced 1,3-dimethyl-1,3-diazinan-2-one.
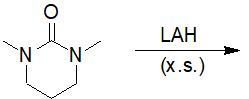
Reactions of Acid Derivatives with Organometallic Reagents
22-17 Predict the product of the following reaction.
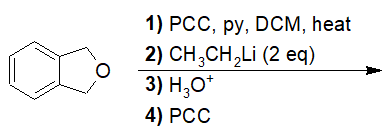
22-18 Choose the correct IUPAC nomenclature of the product of the following reaction.
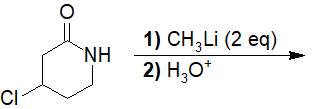
a) 6-amino-5-chlorohexan-2-one
b) 6-amino-5-chloro-2-methylhexan-2-ol
c) 6-amino-4-chloro-2-methylhexan-2-ol
d) 4-chloro-2-methylpiperidin-2-ol
22-19 Decide whether or not the following reaction is the best way to obtain the final product. If not, suggest a better route of synthesis.