Structural and Geometric Isomerism
4-1
a) There are five alkane isomers of hexane C6H14. Draw and name all of them.
b) The heat of combustion of hexane is 4163.2 kJ/mol. Heat of combustion of neohexane is 4159.5 kJ/mol. Predict the relative stability of these two compounds?
c) Draw and name all cycloalkane isomers of C5H10, including all possible geometric (cis-trans) stereoisomers.
4-2 Which of the following structures represent the same compound? Name the structures given in part (a), (d), (e), (f), (g)
a) 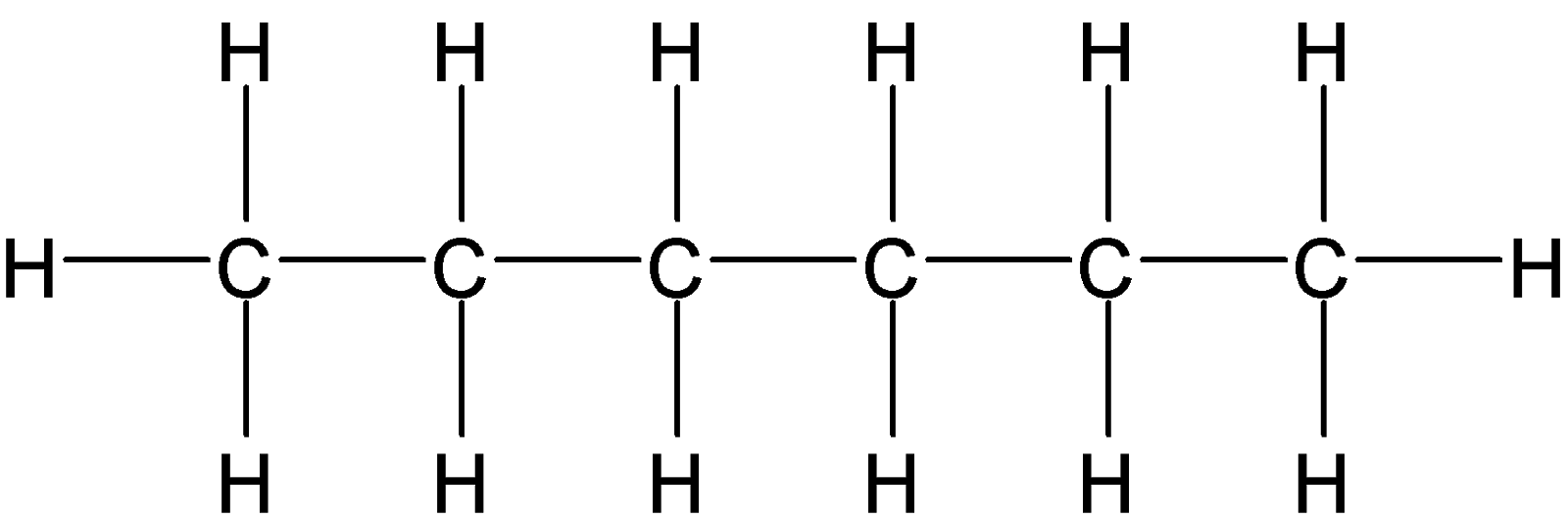
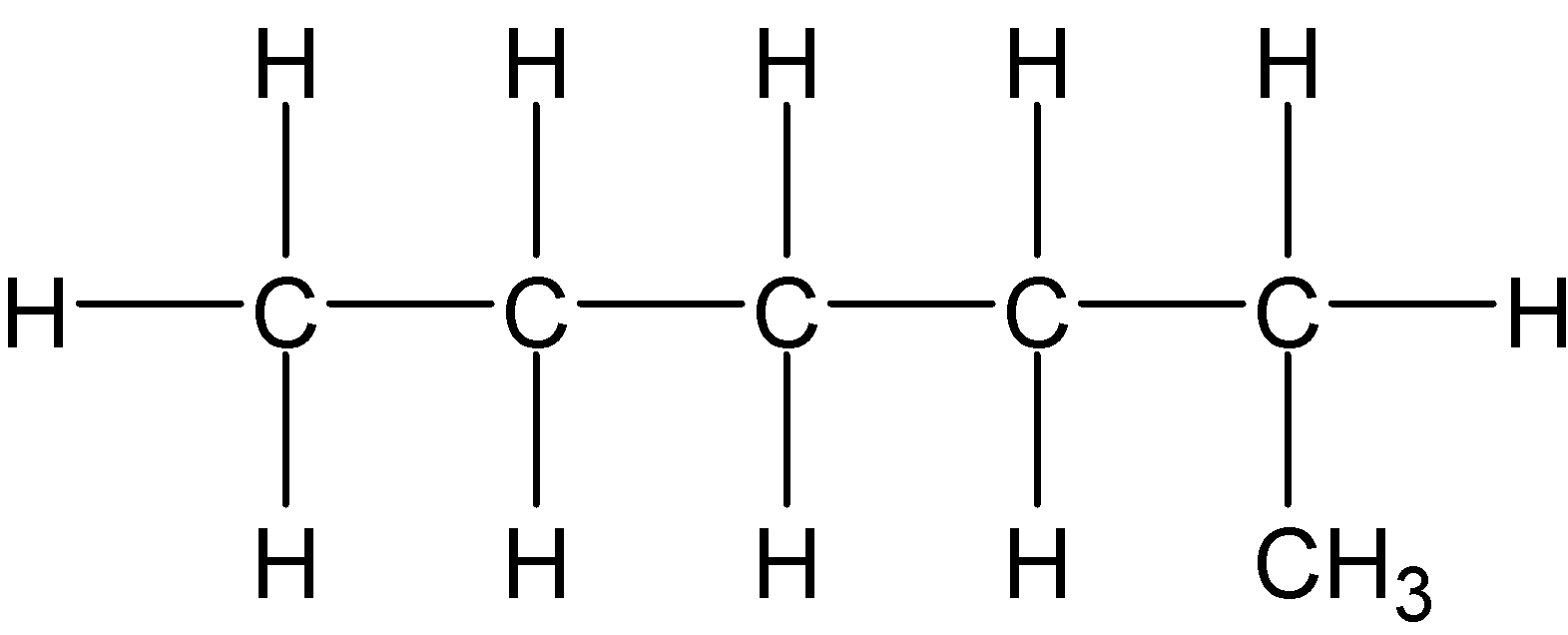
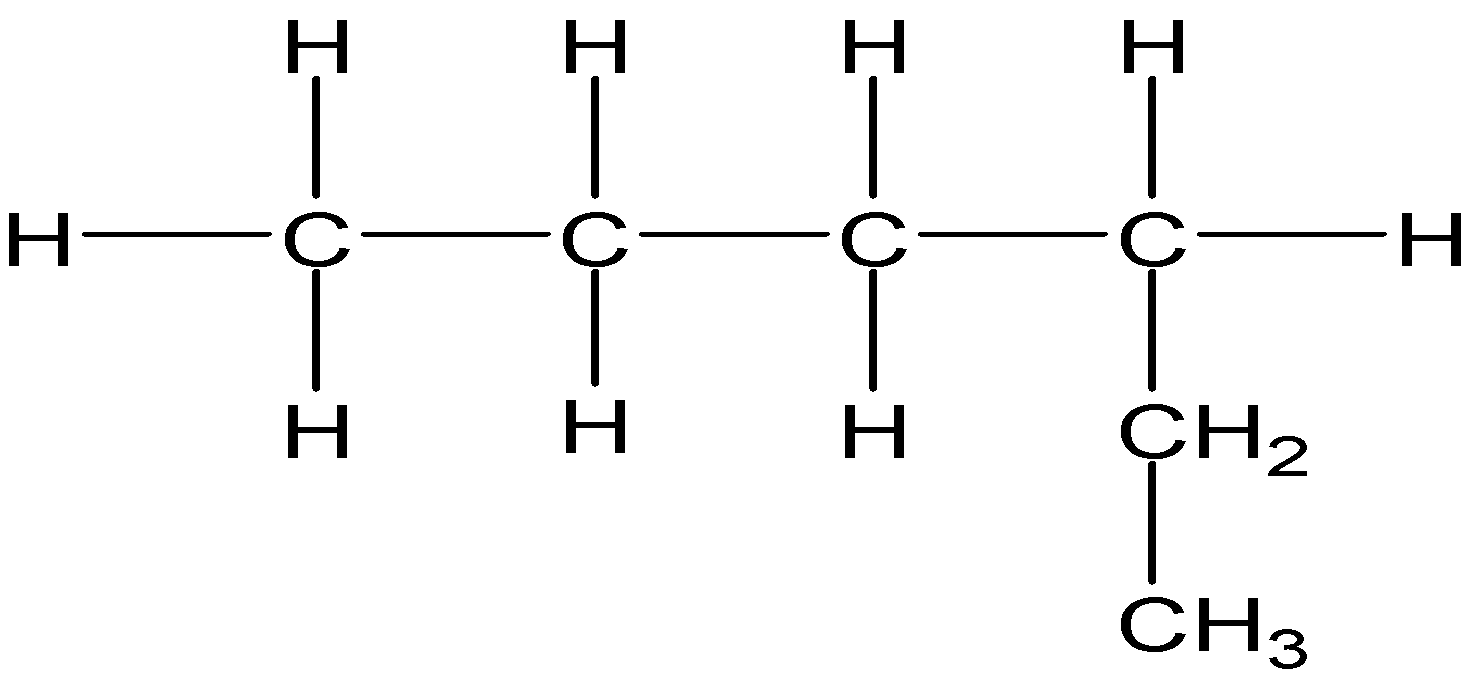
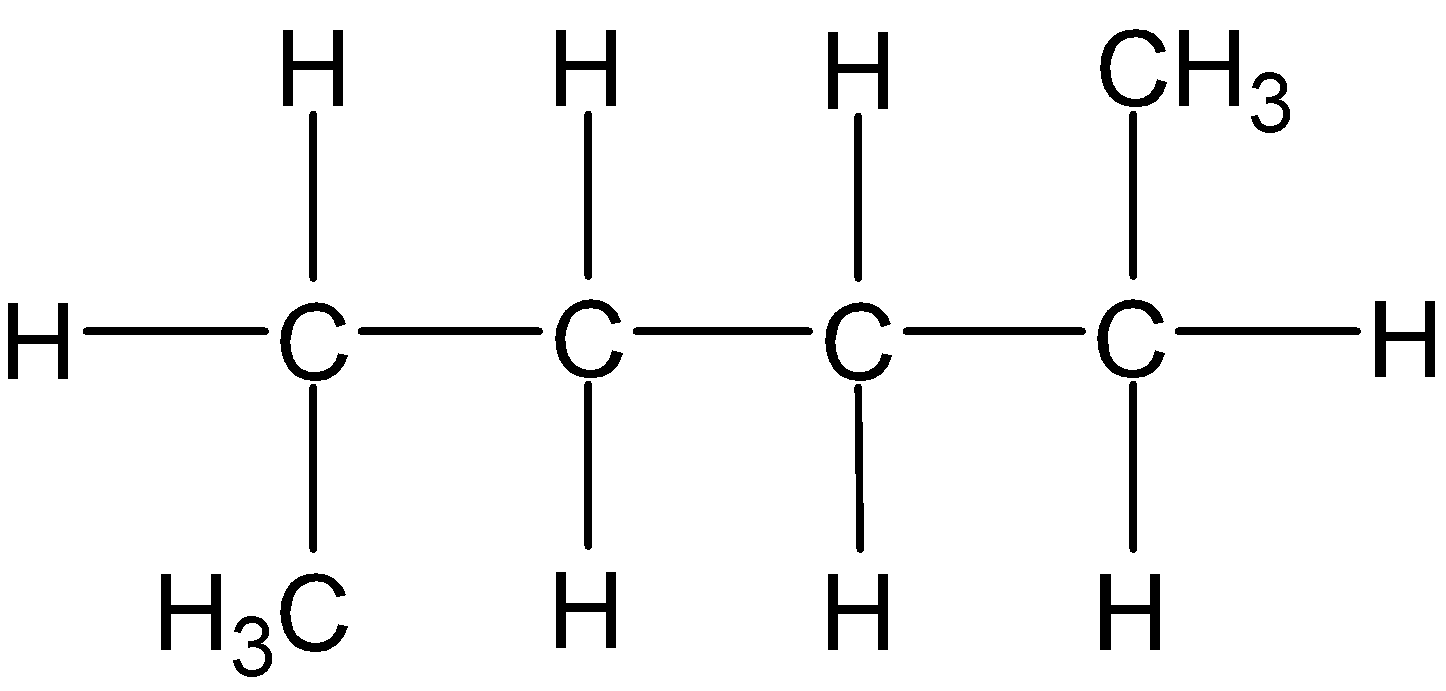
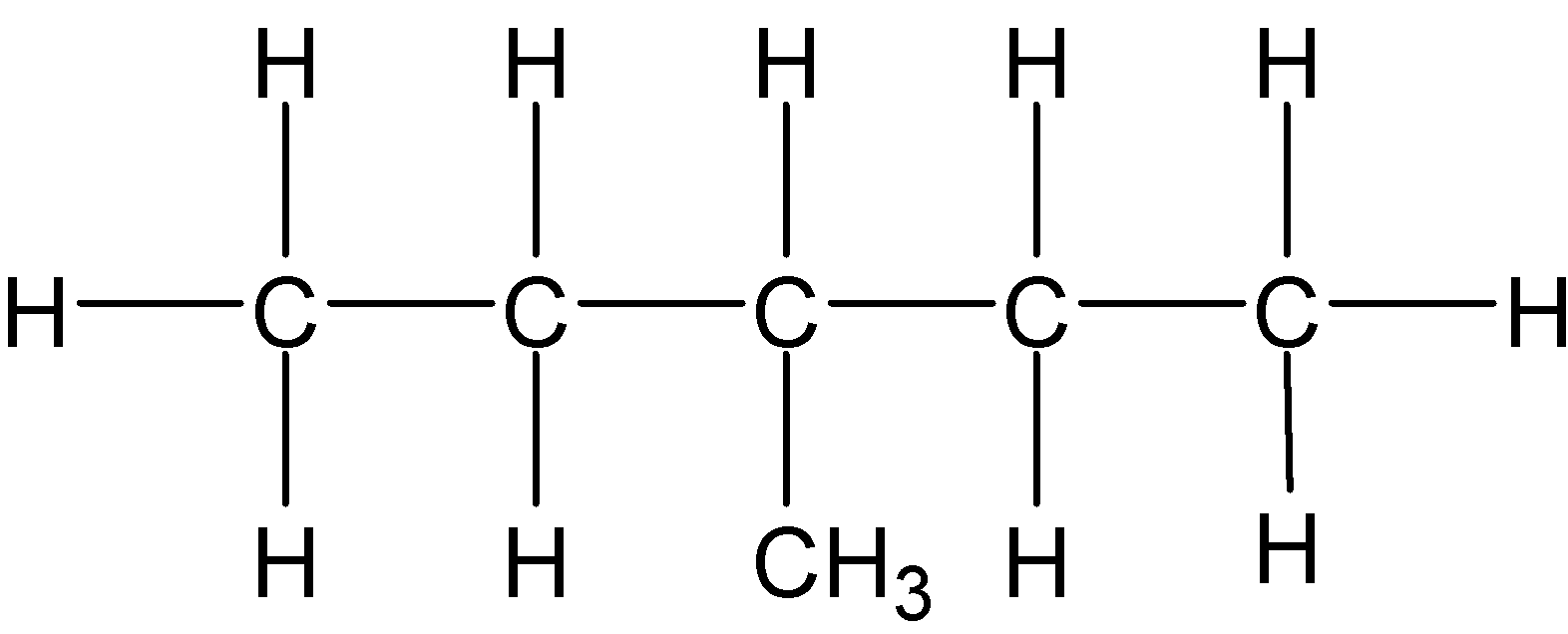
b) 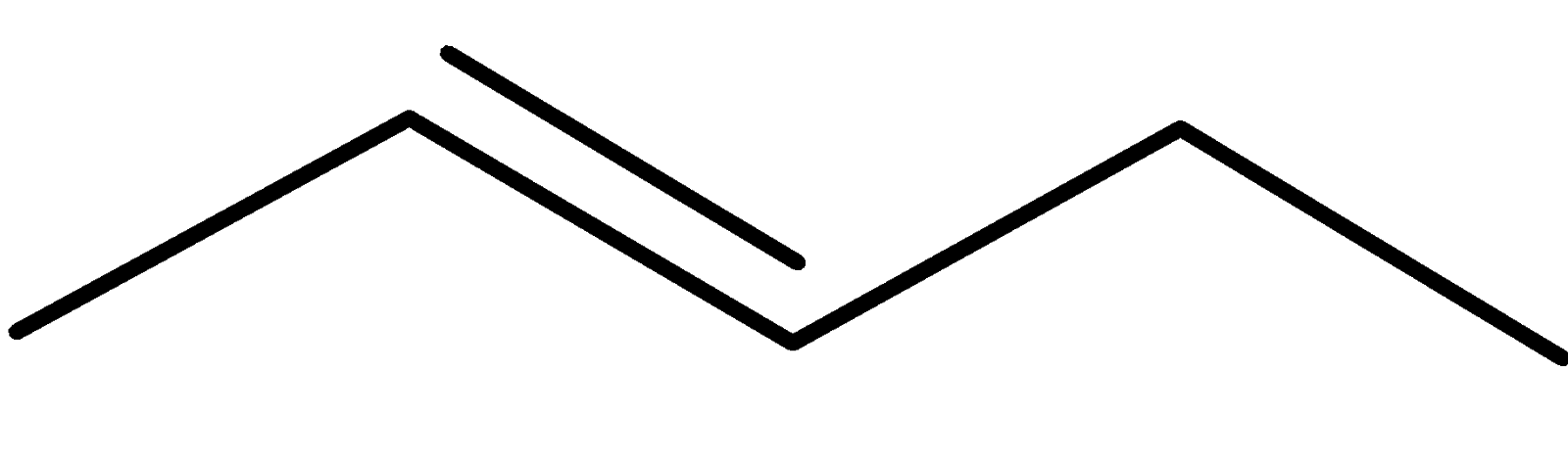
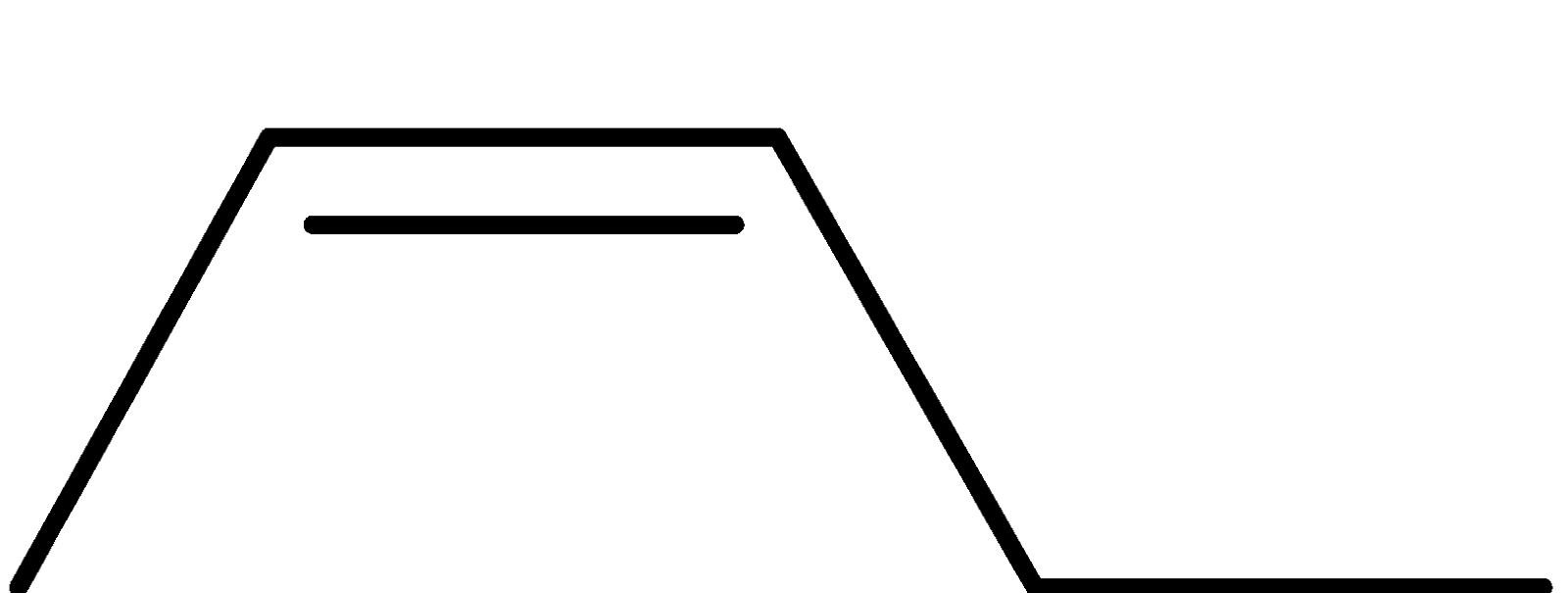
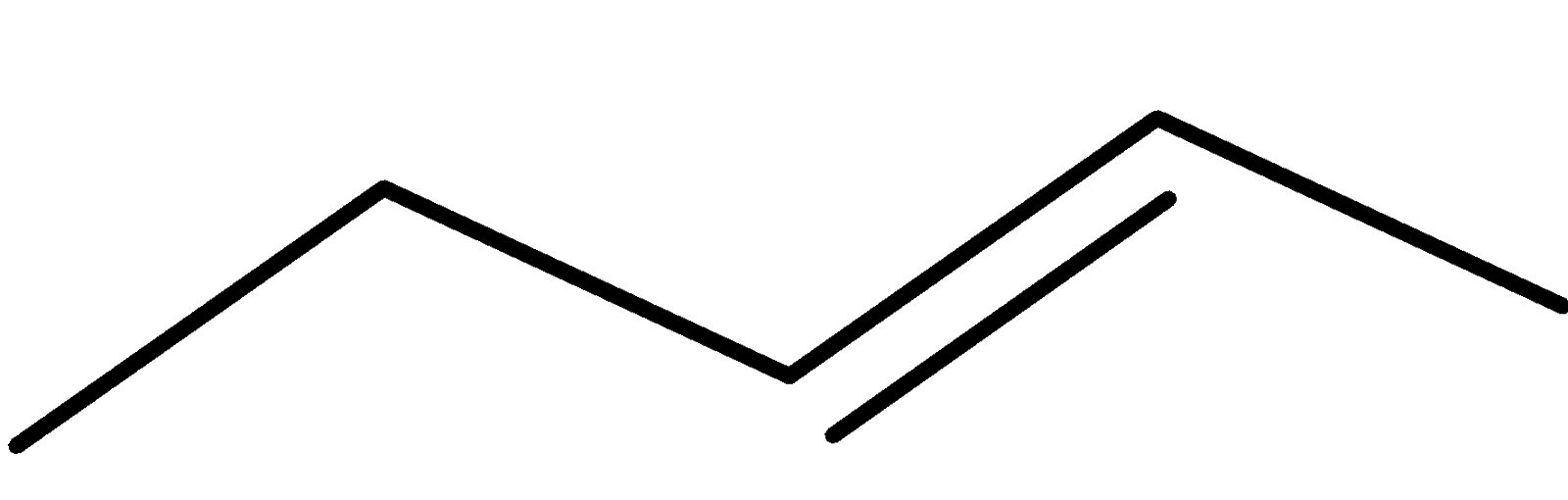
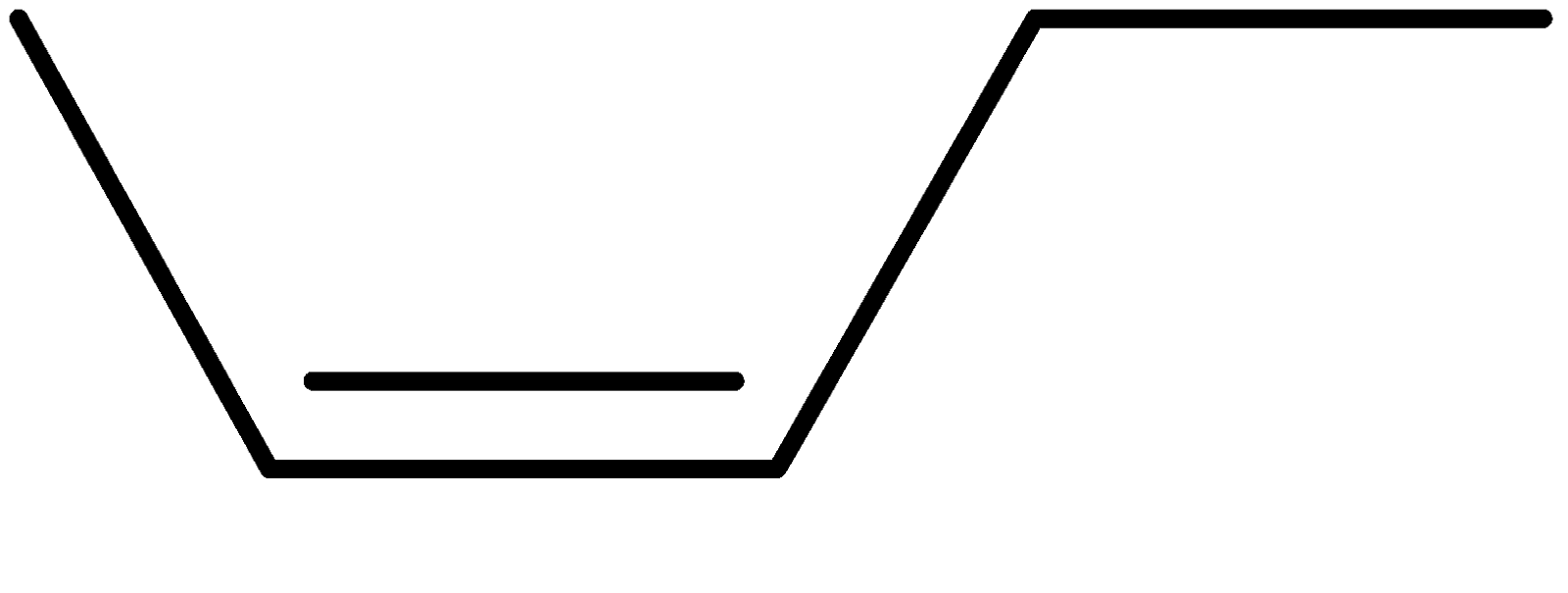
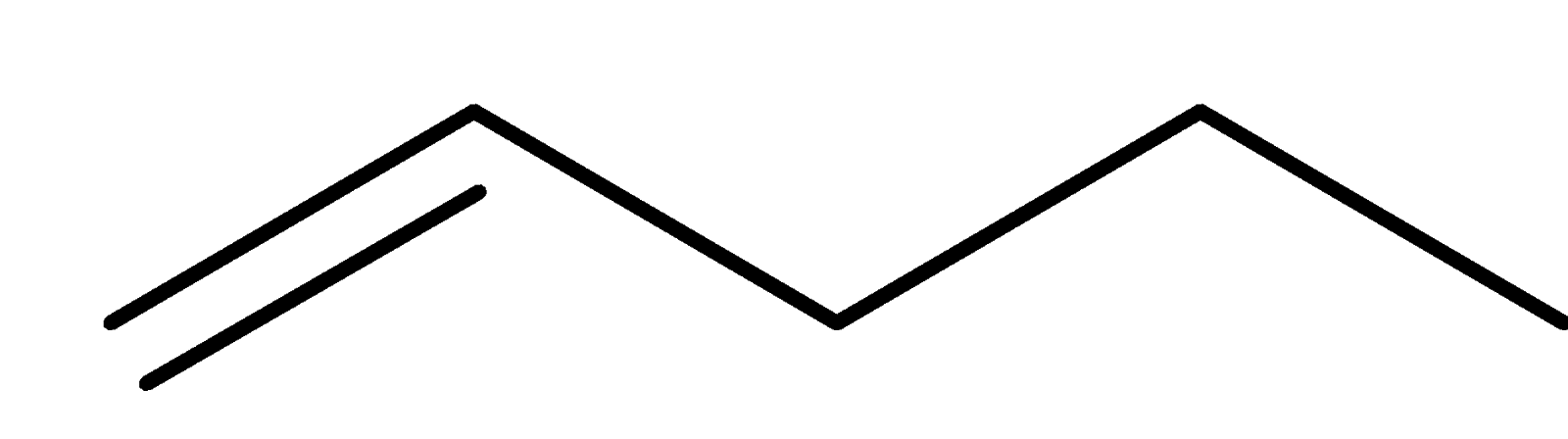
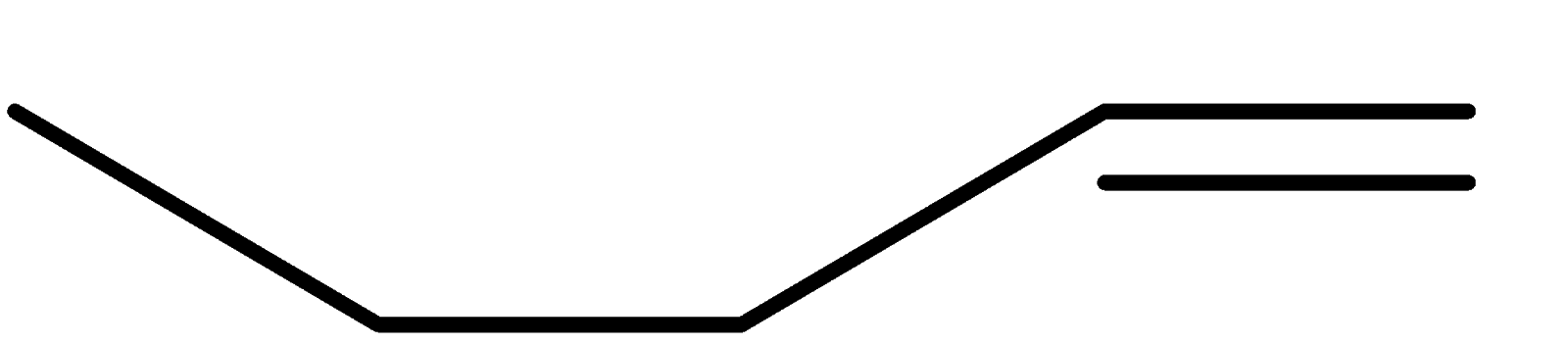
c) 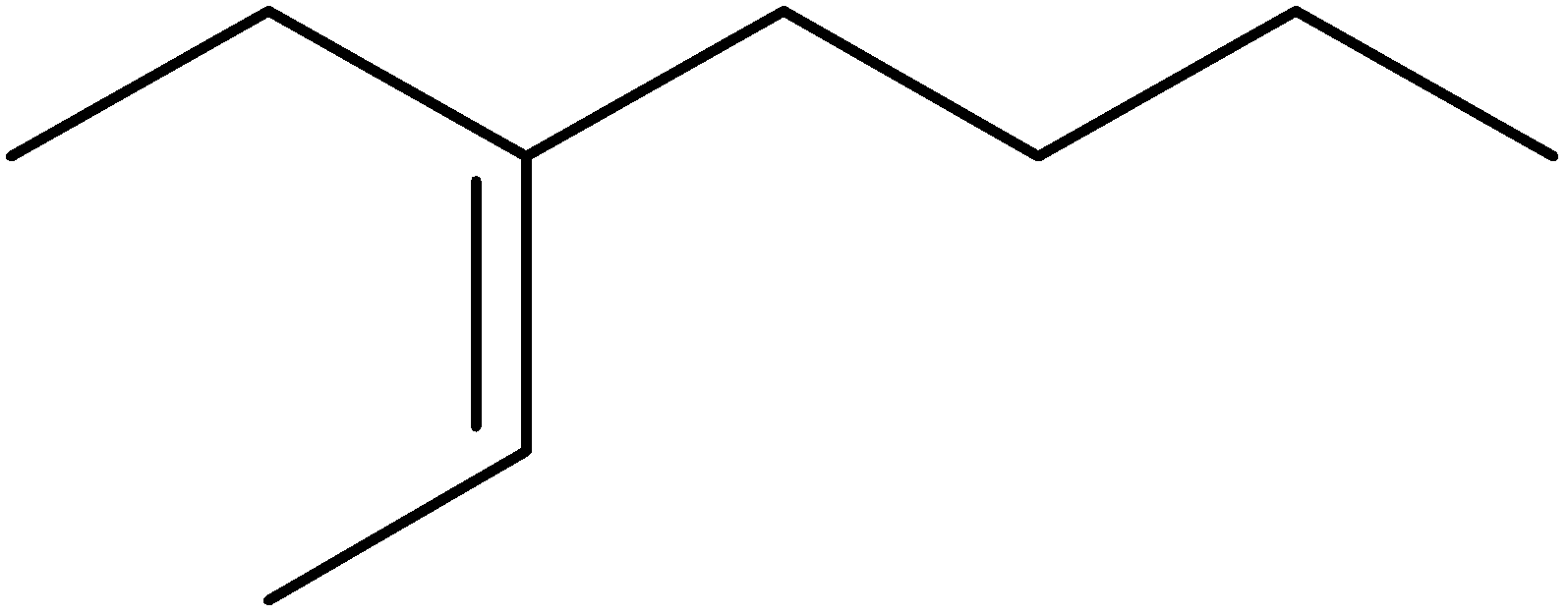
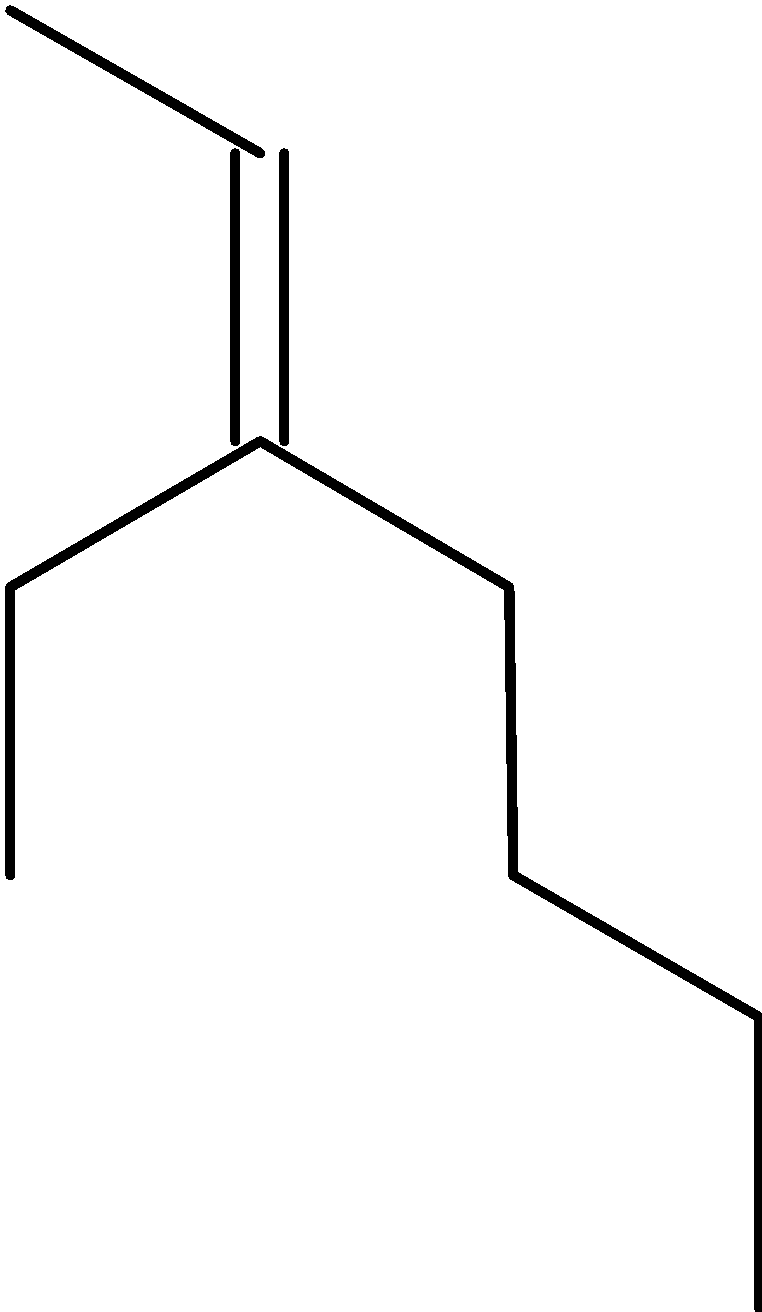
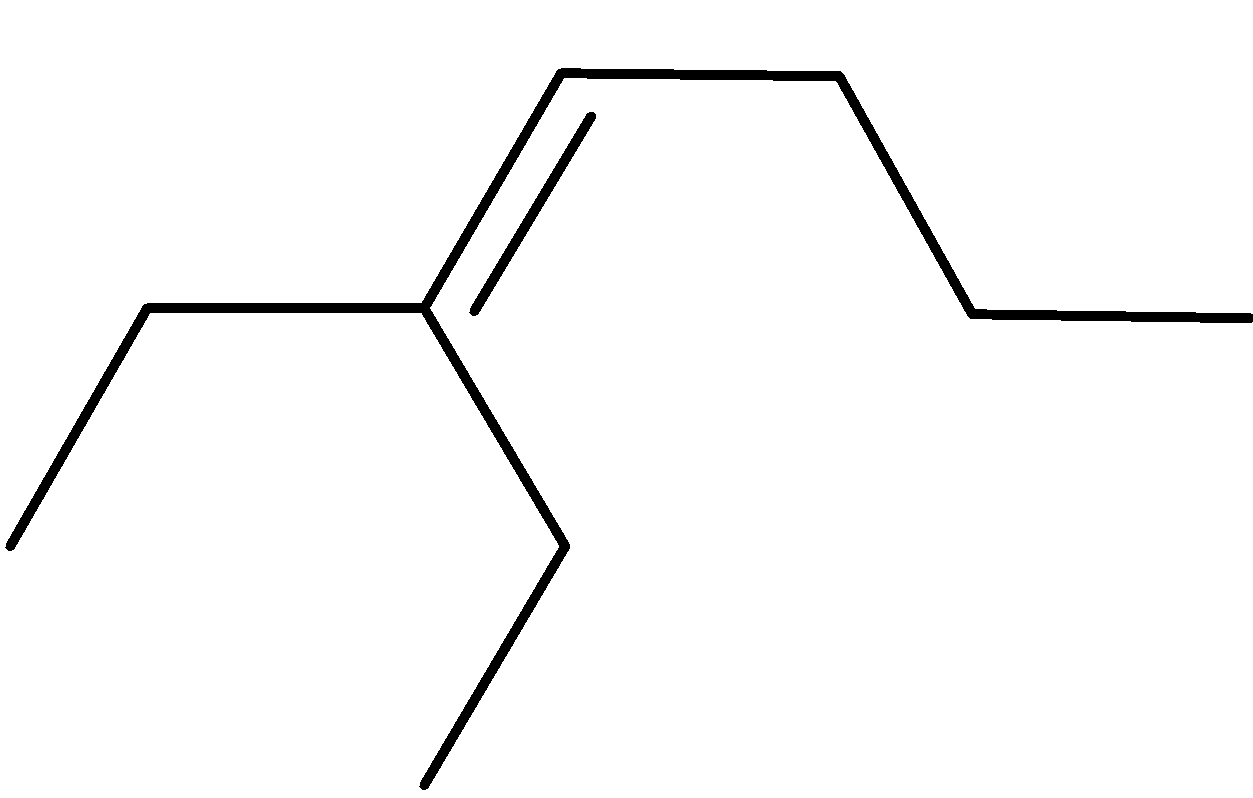
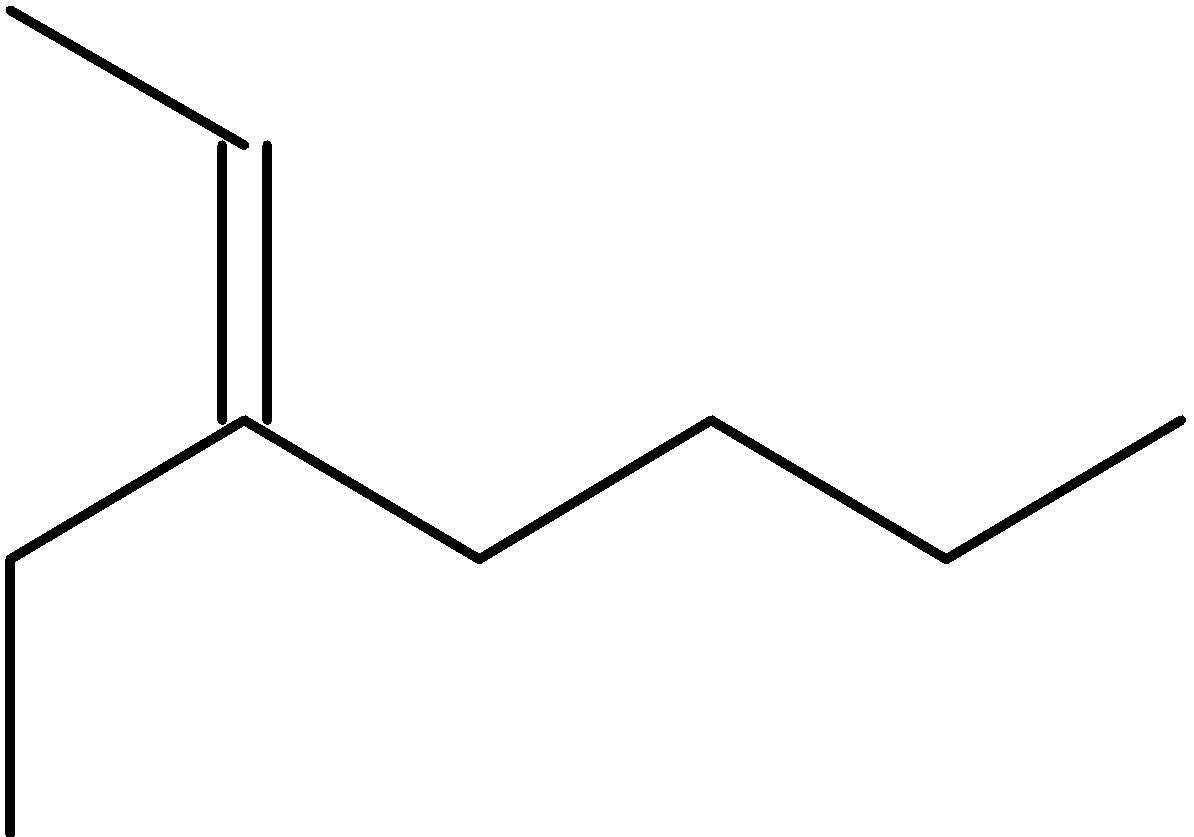
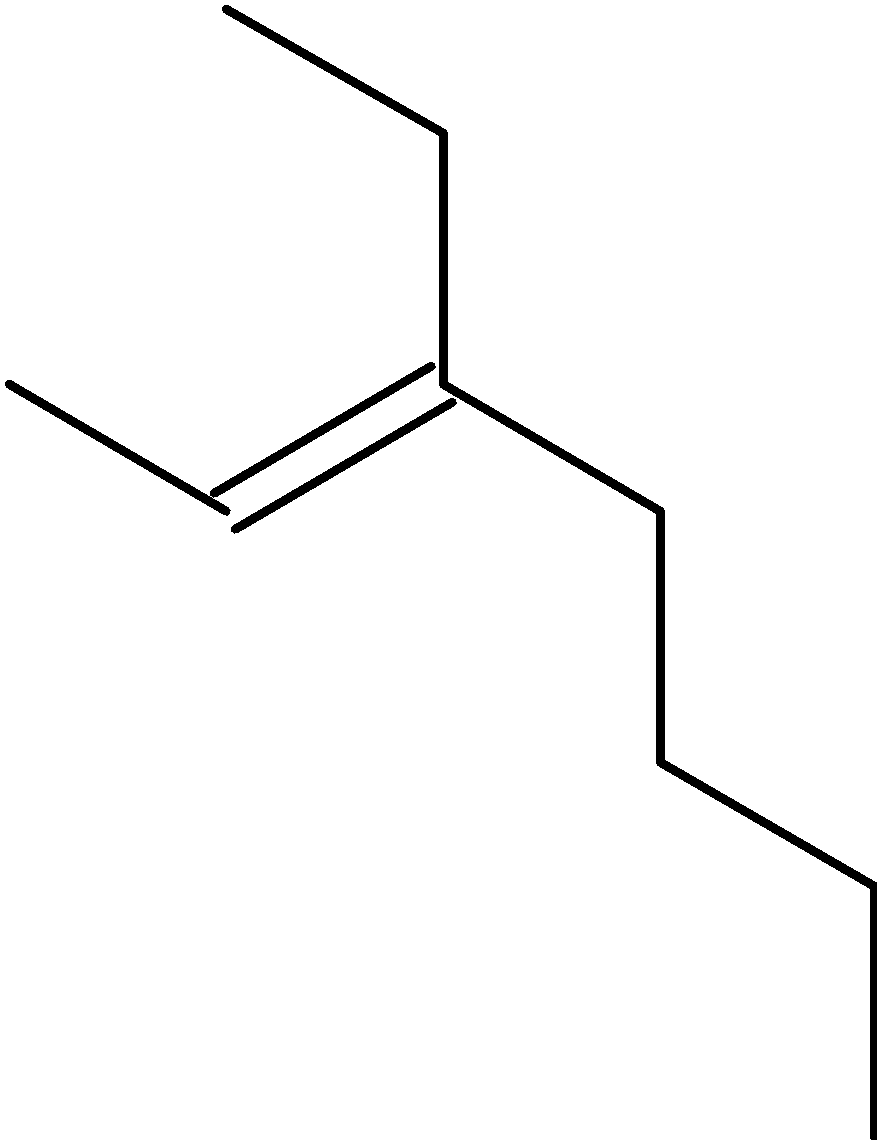
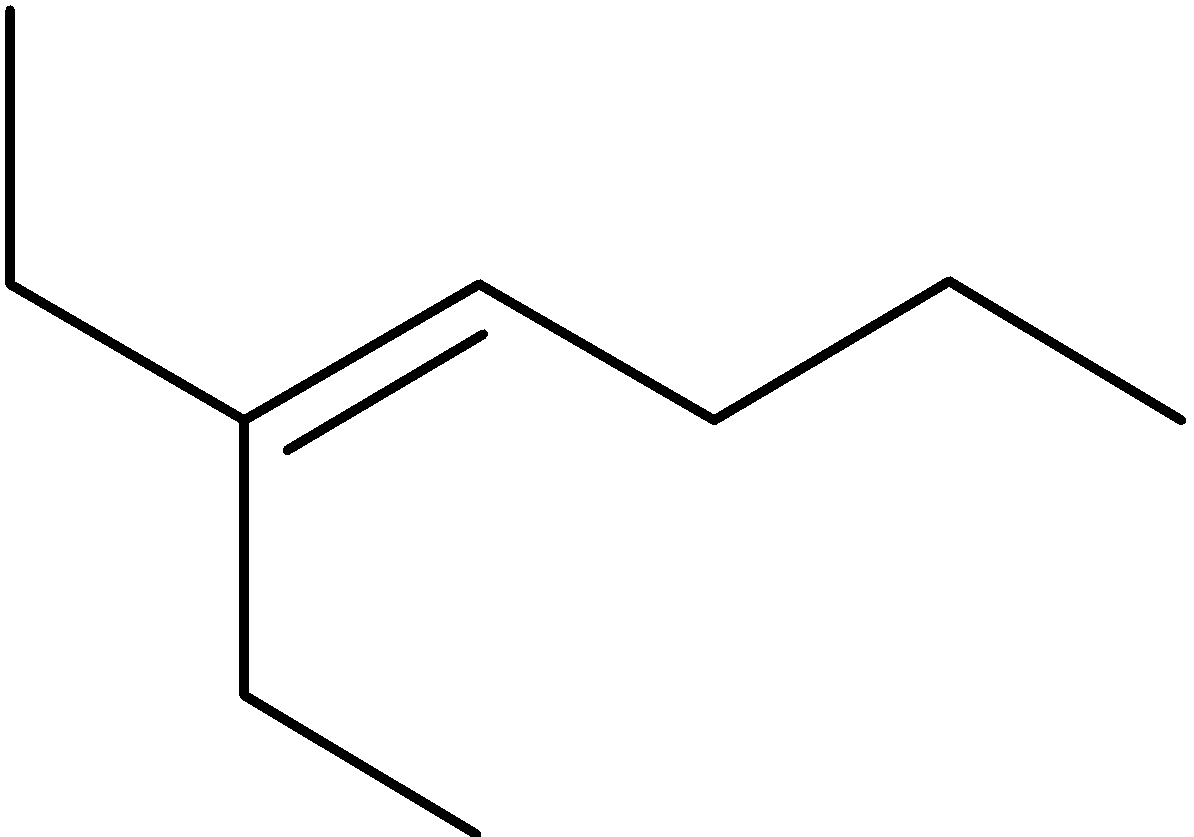
d) 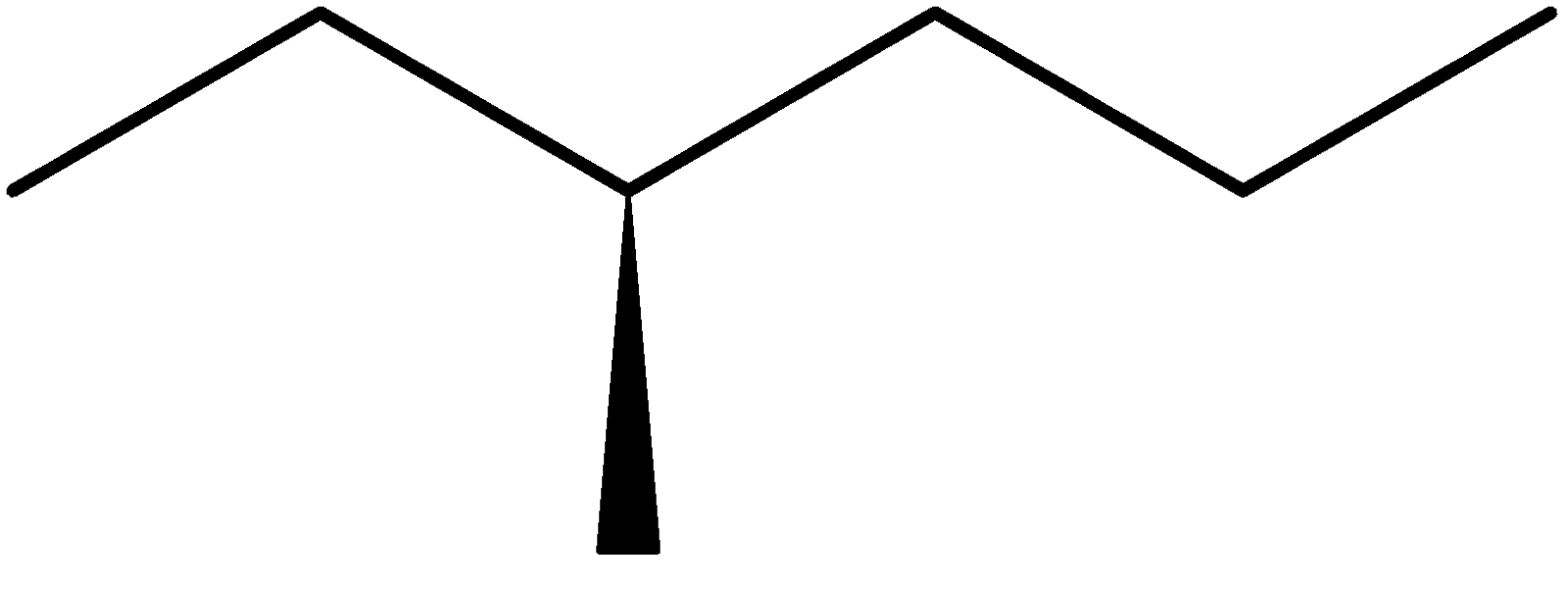
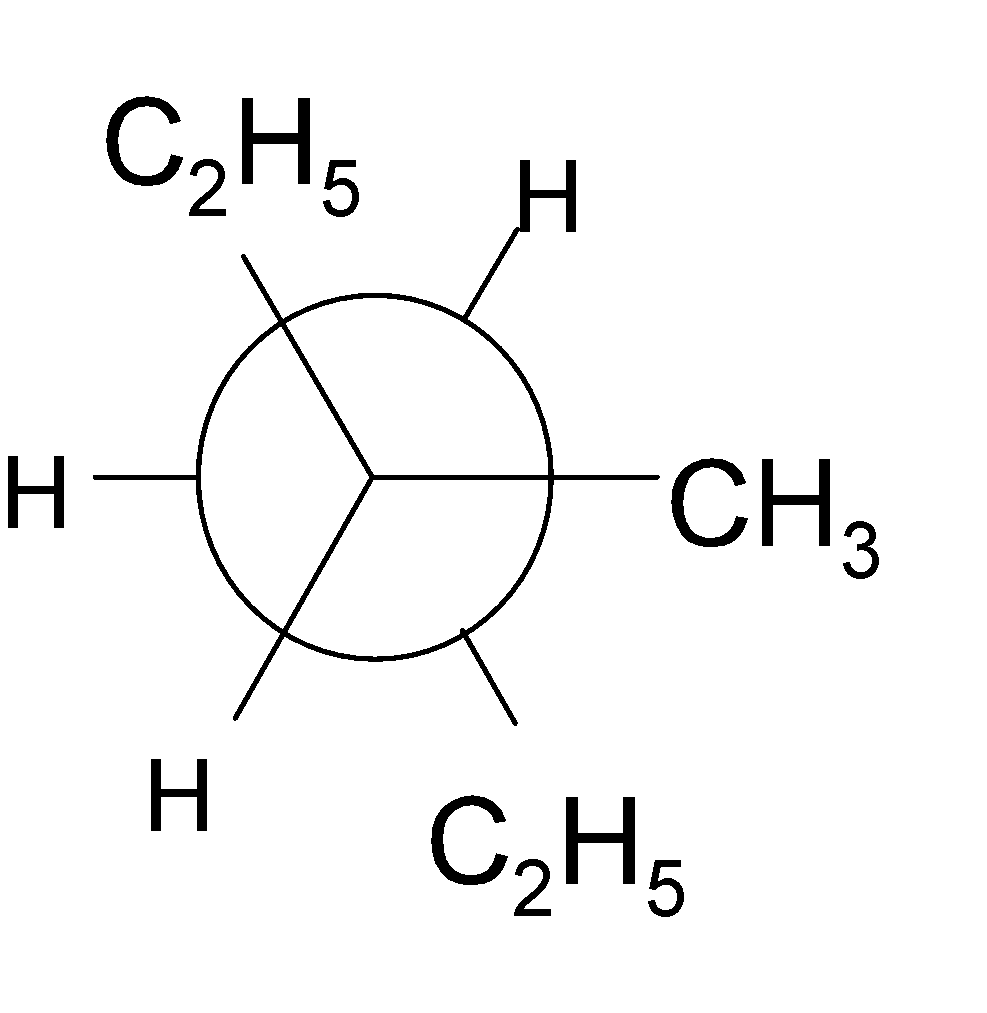

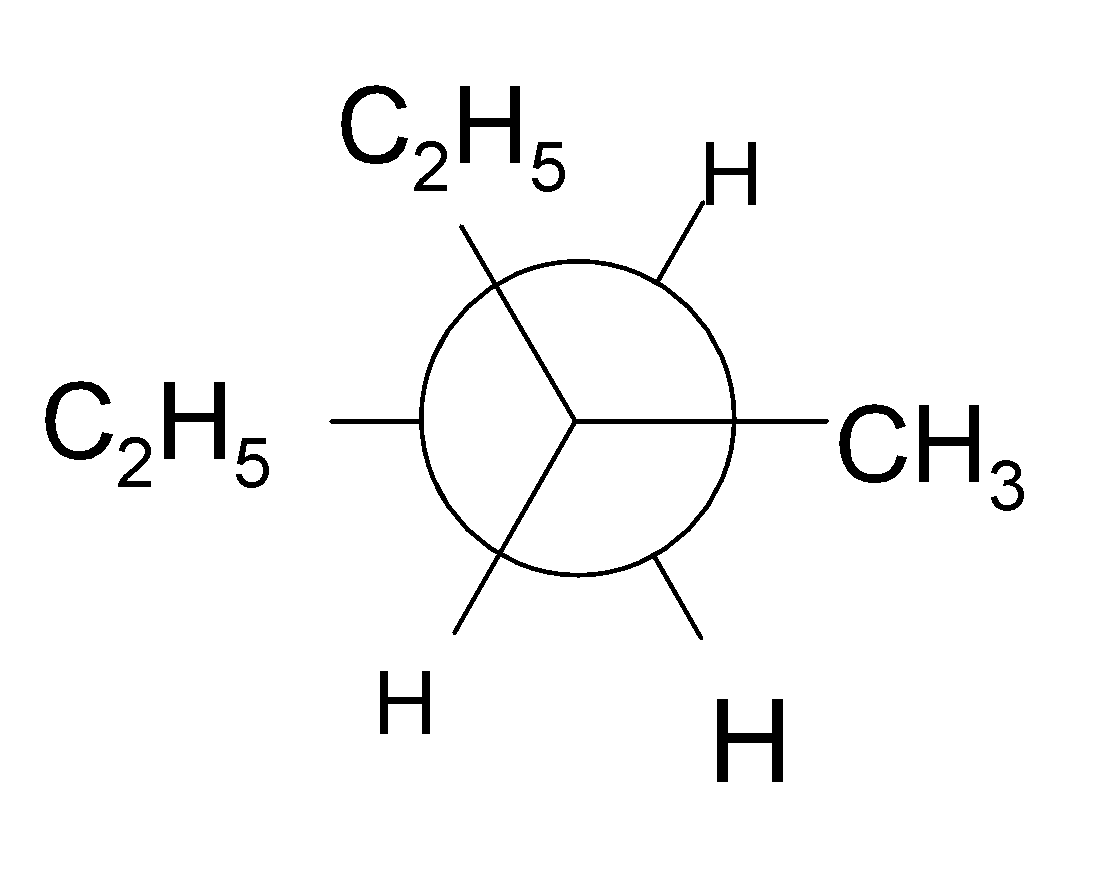
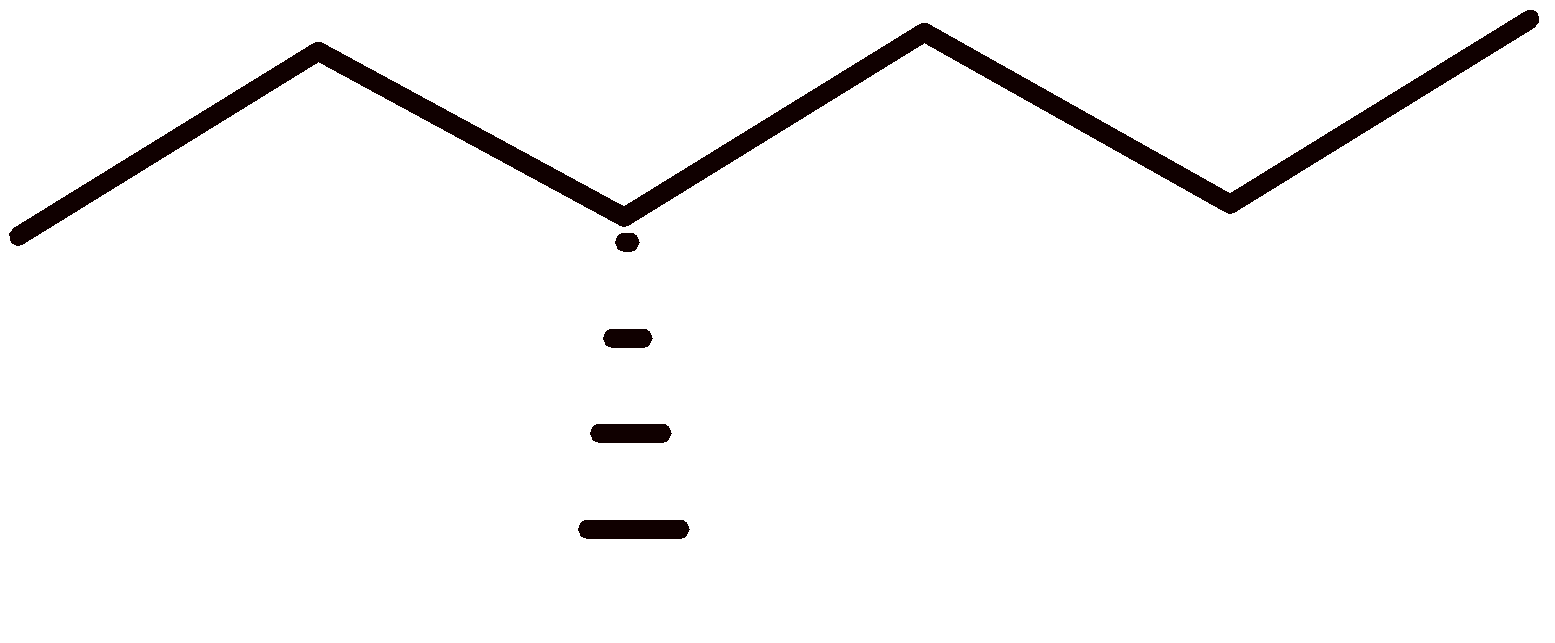
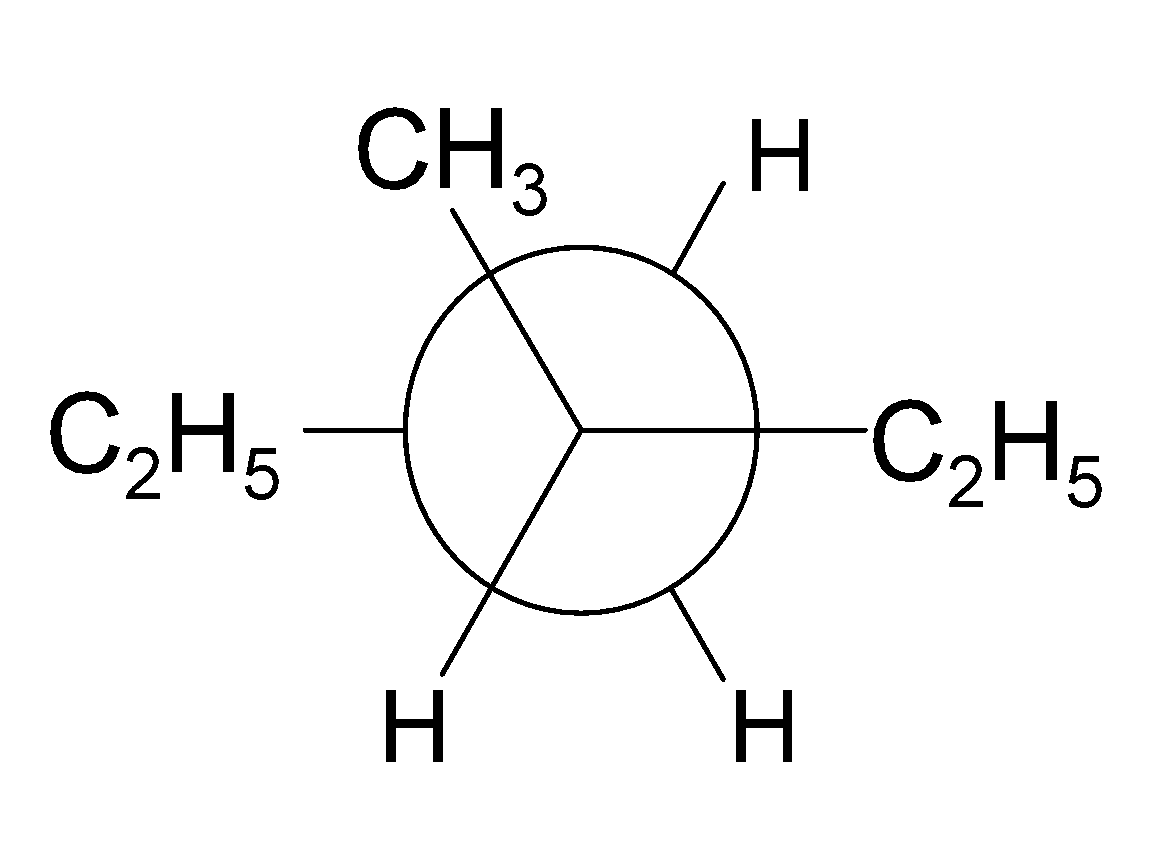
e) 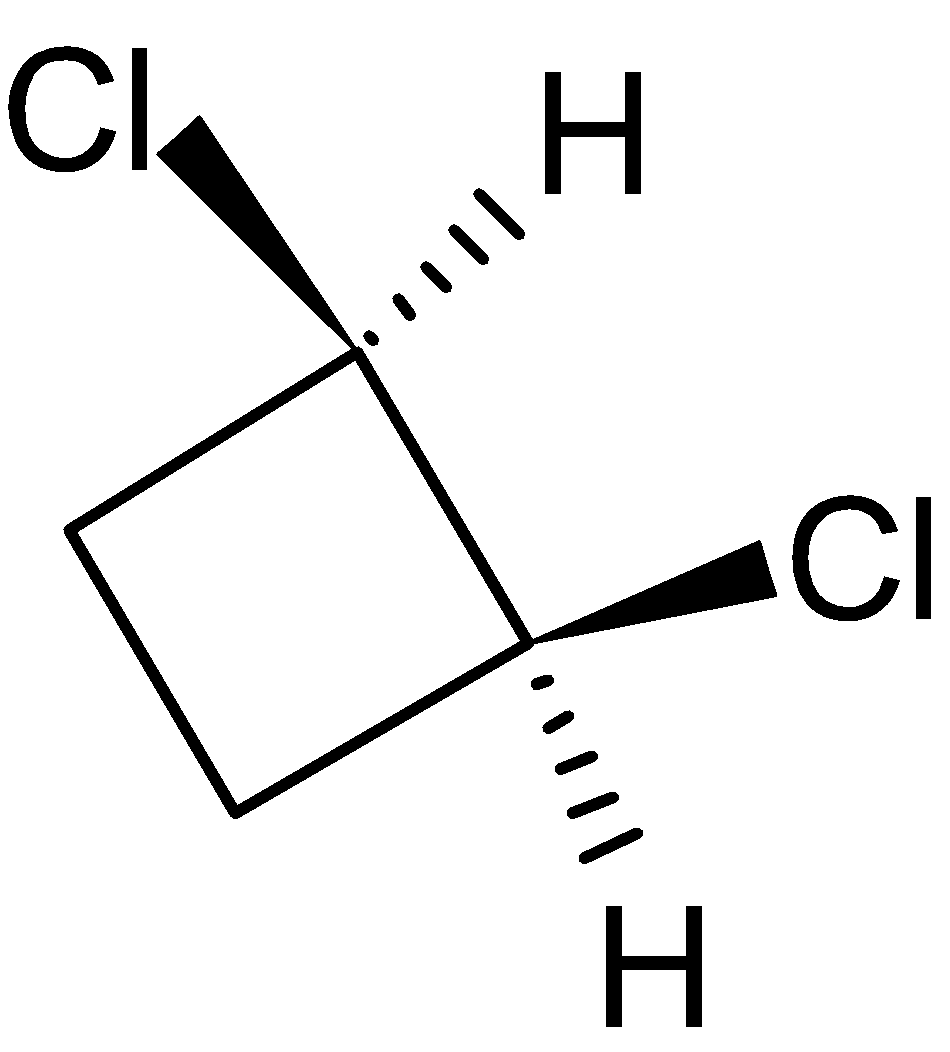
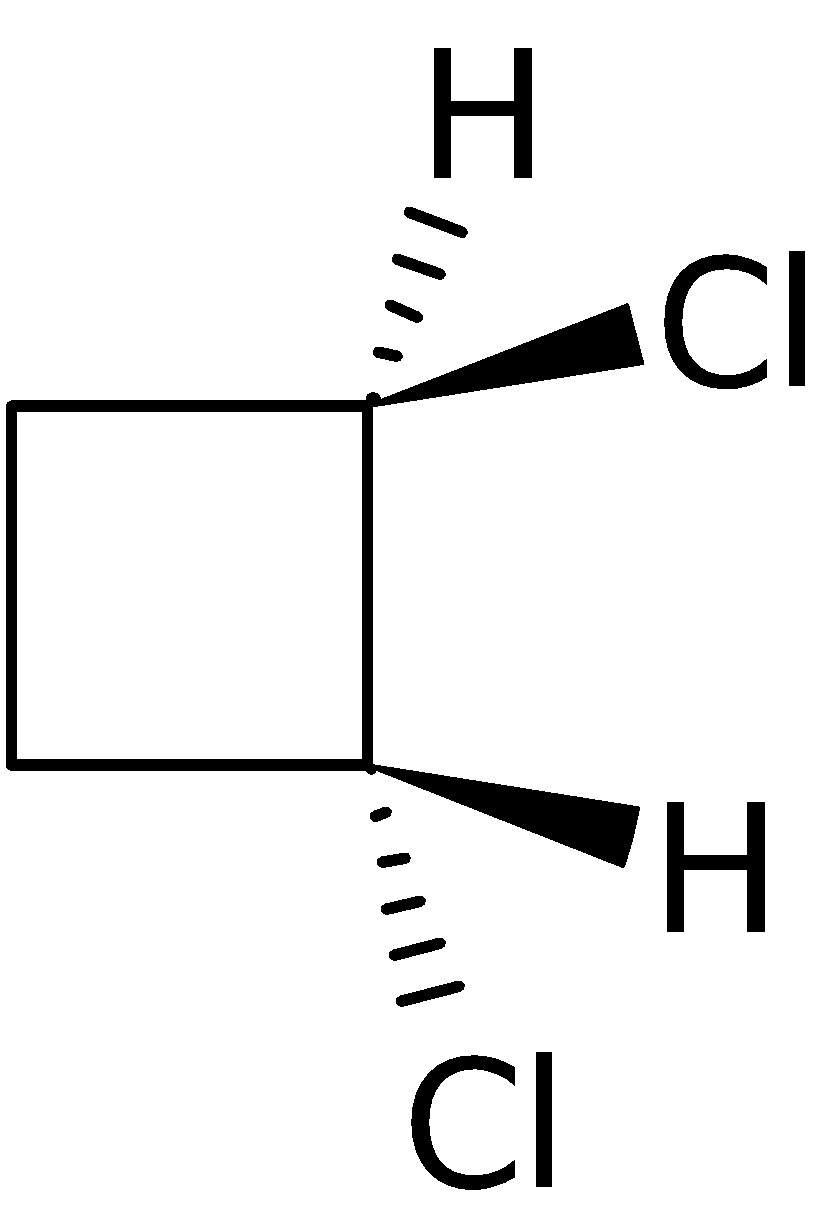
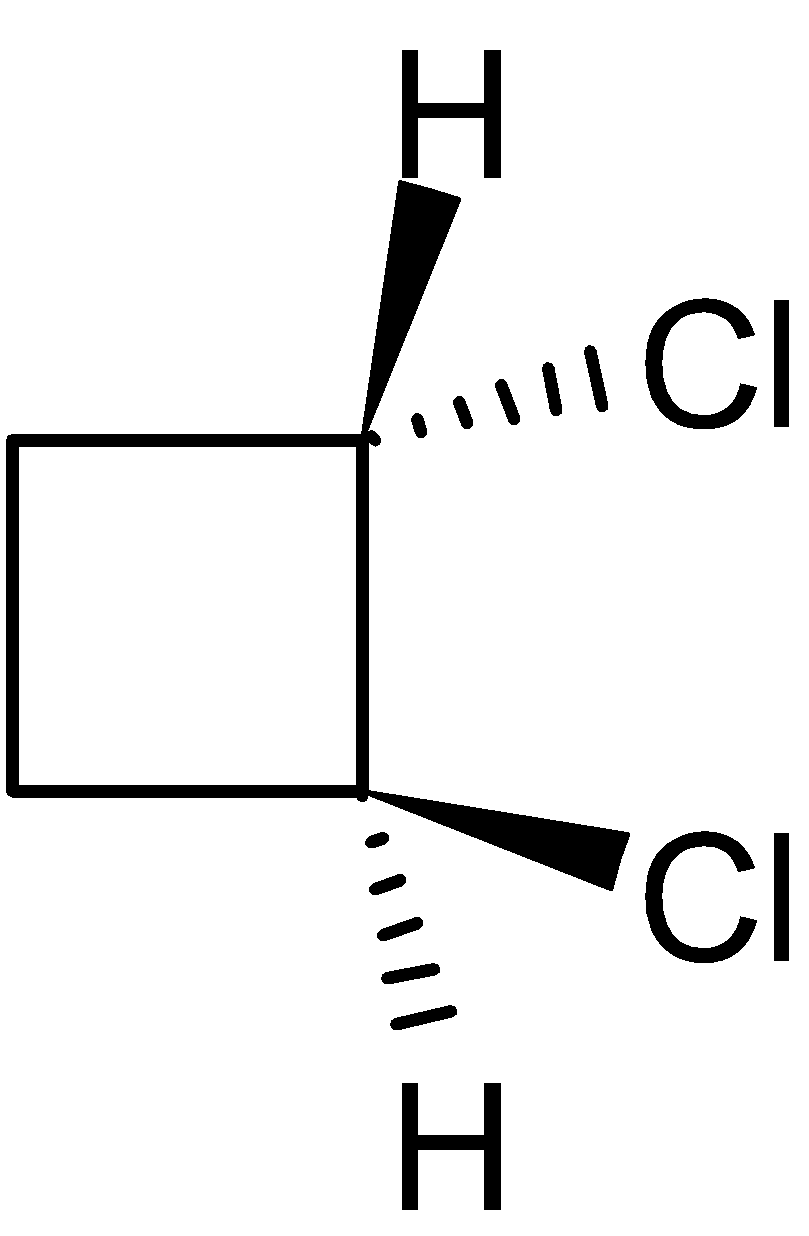
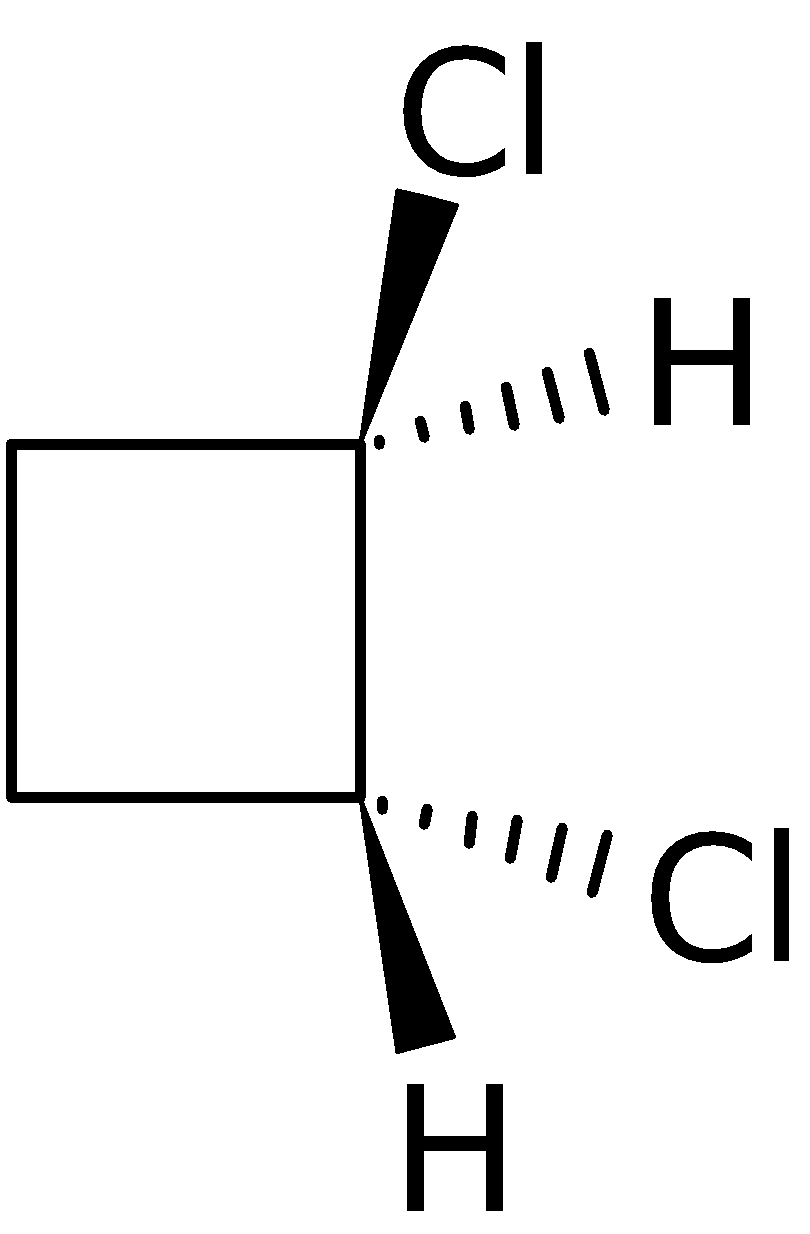
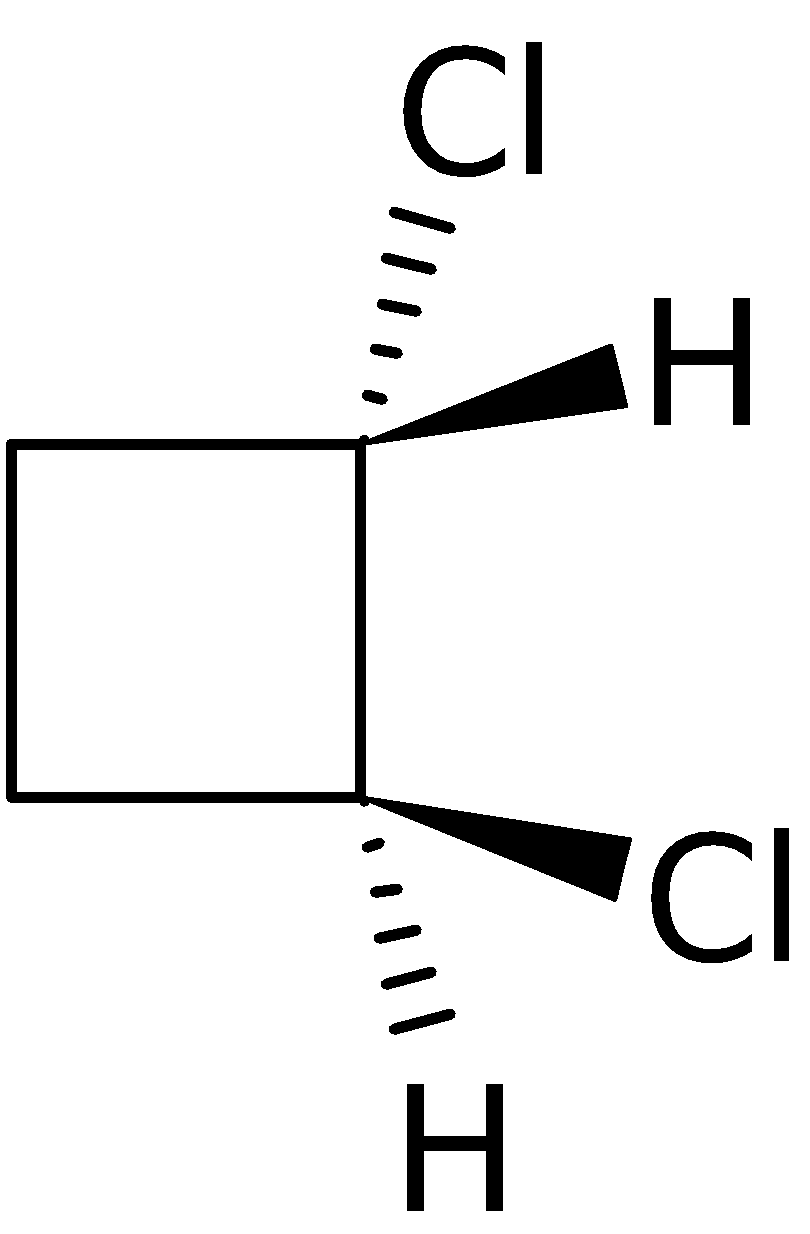
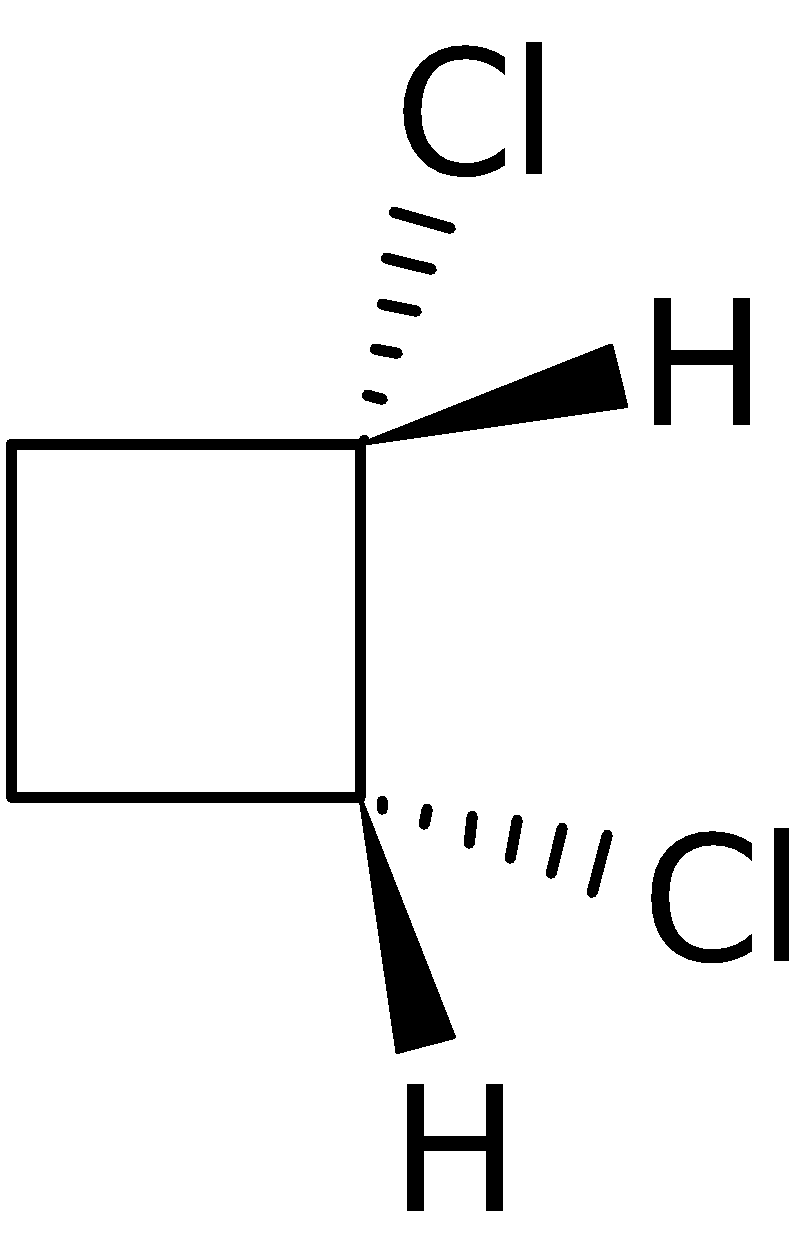
f) 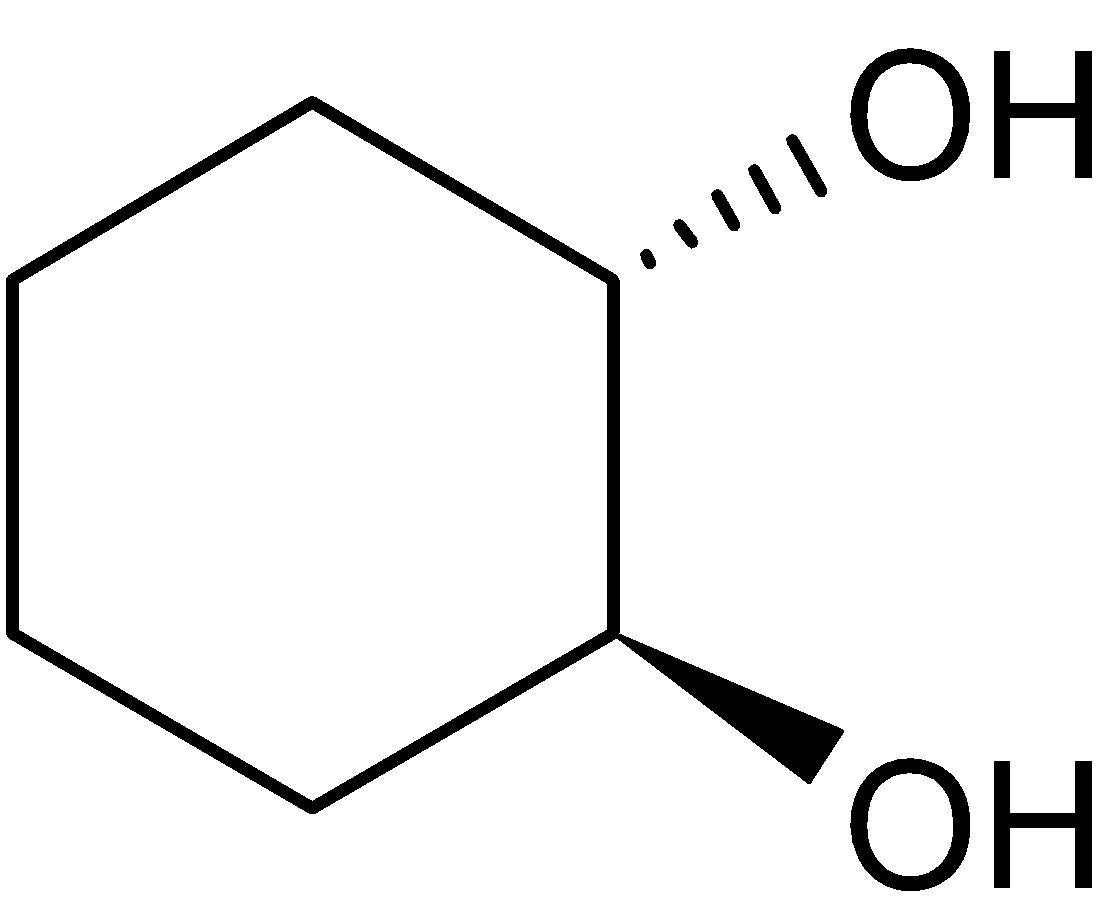

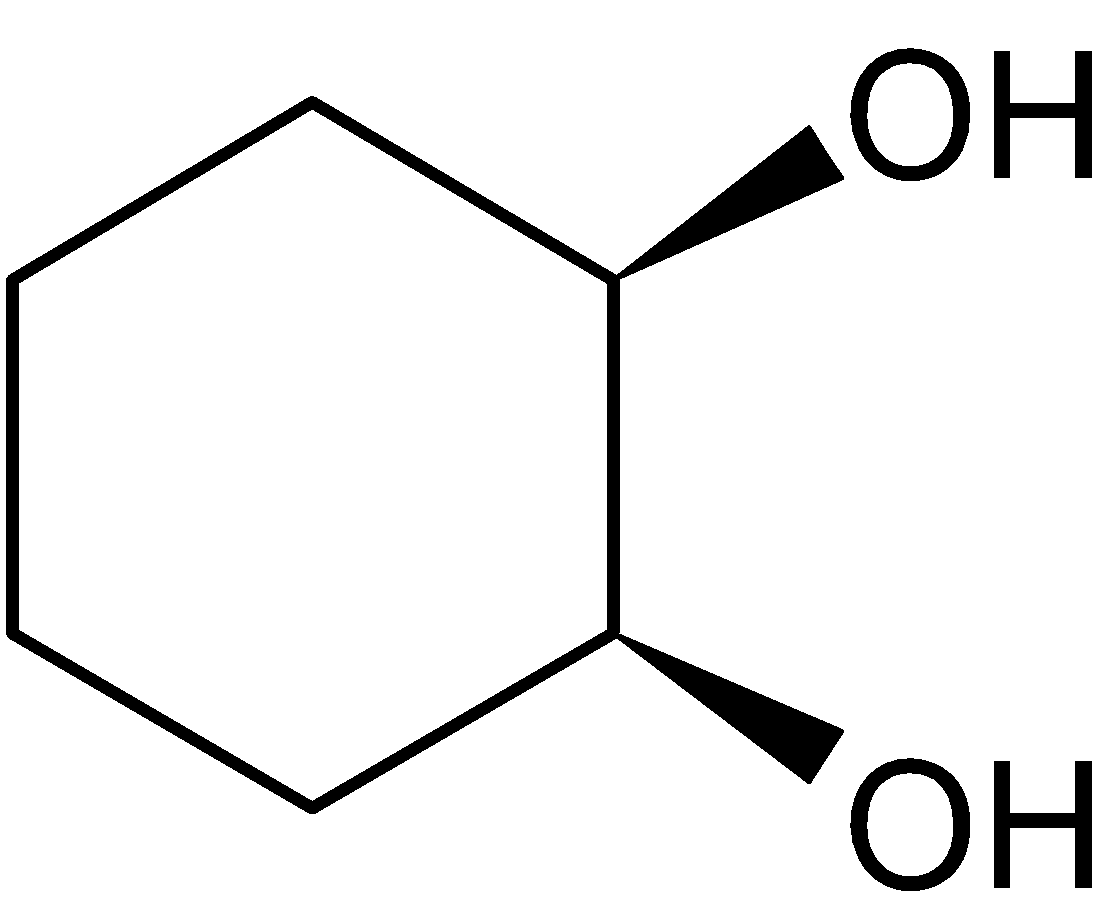

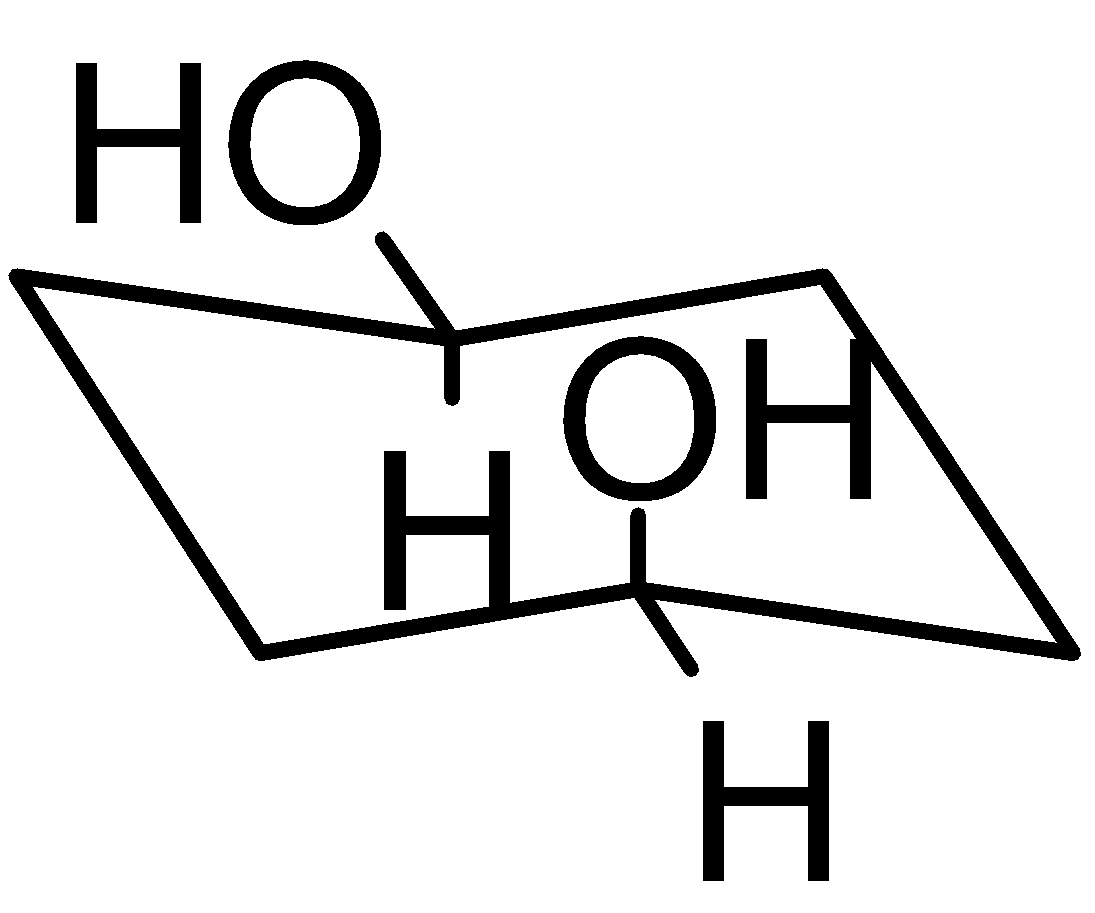
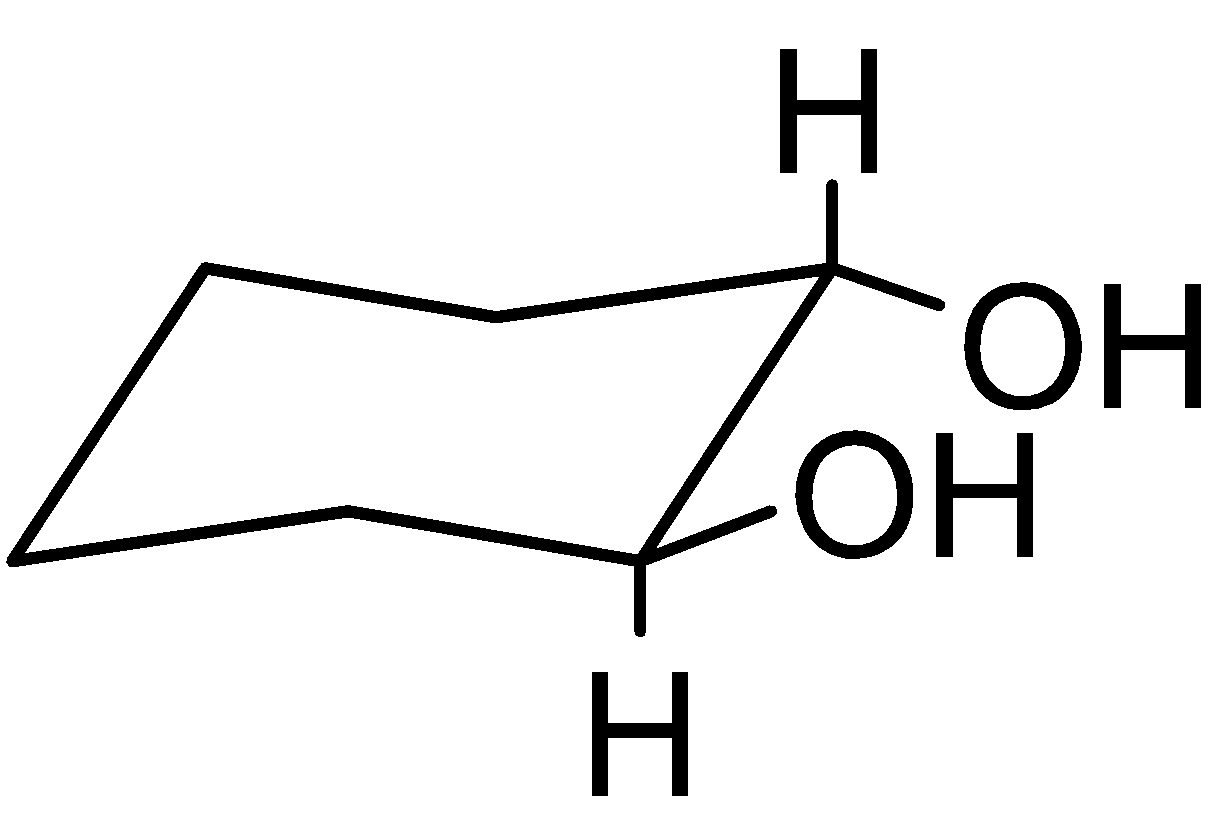
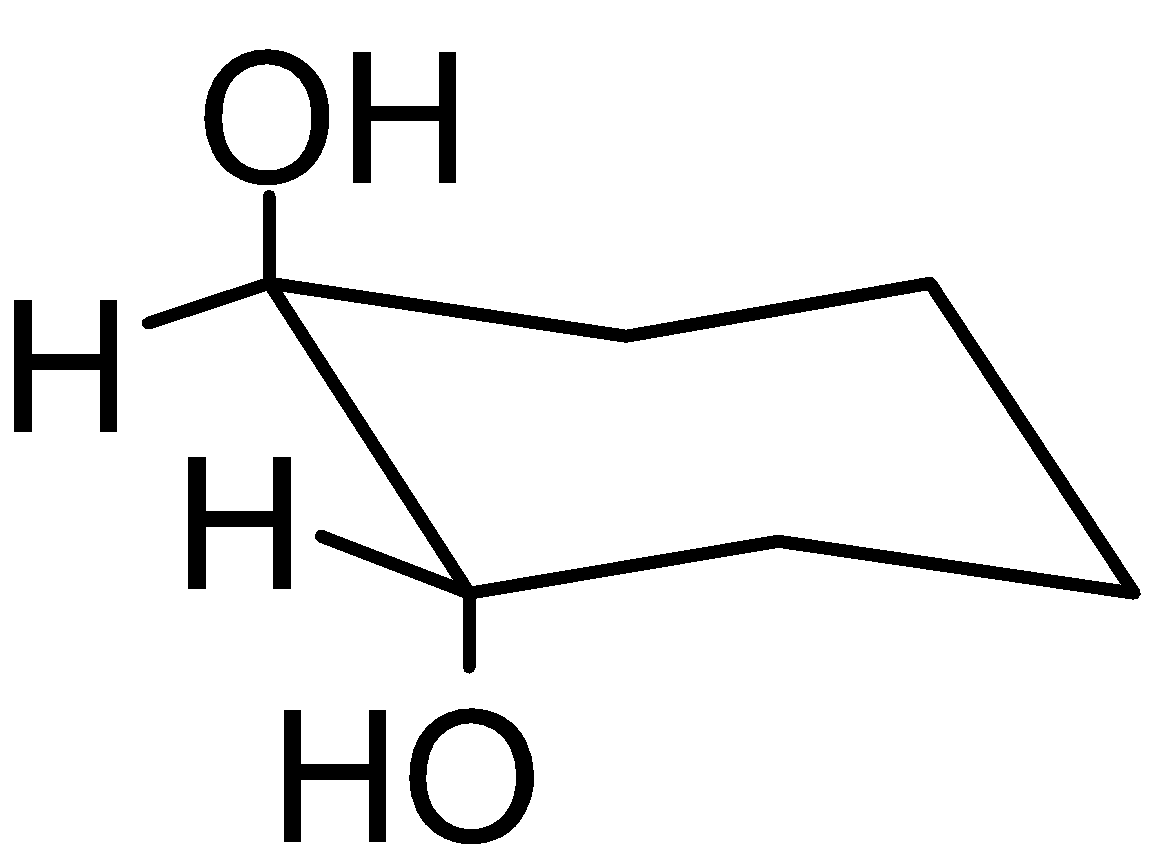
g) 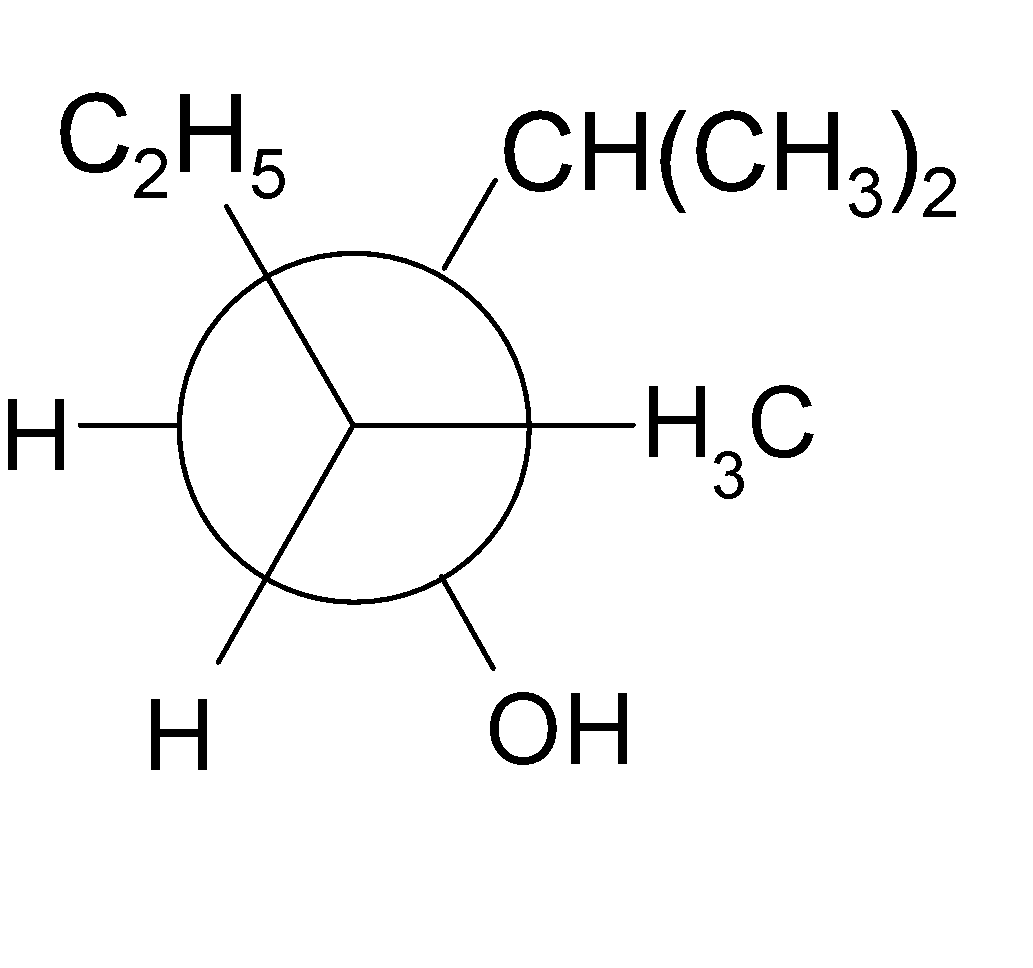
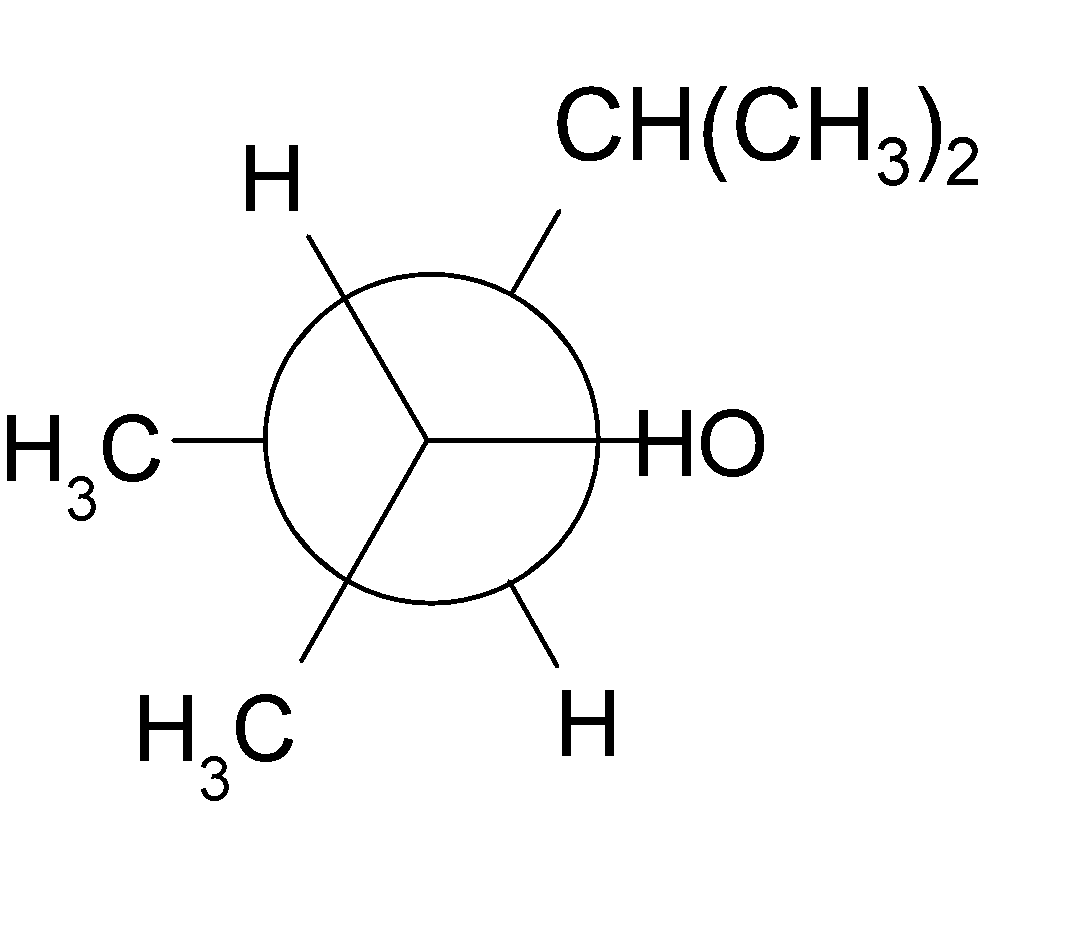
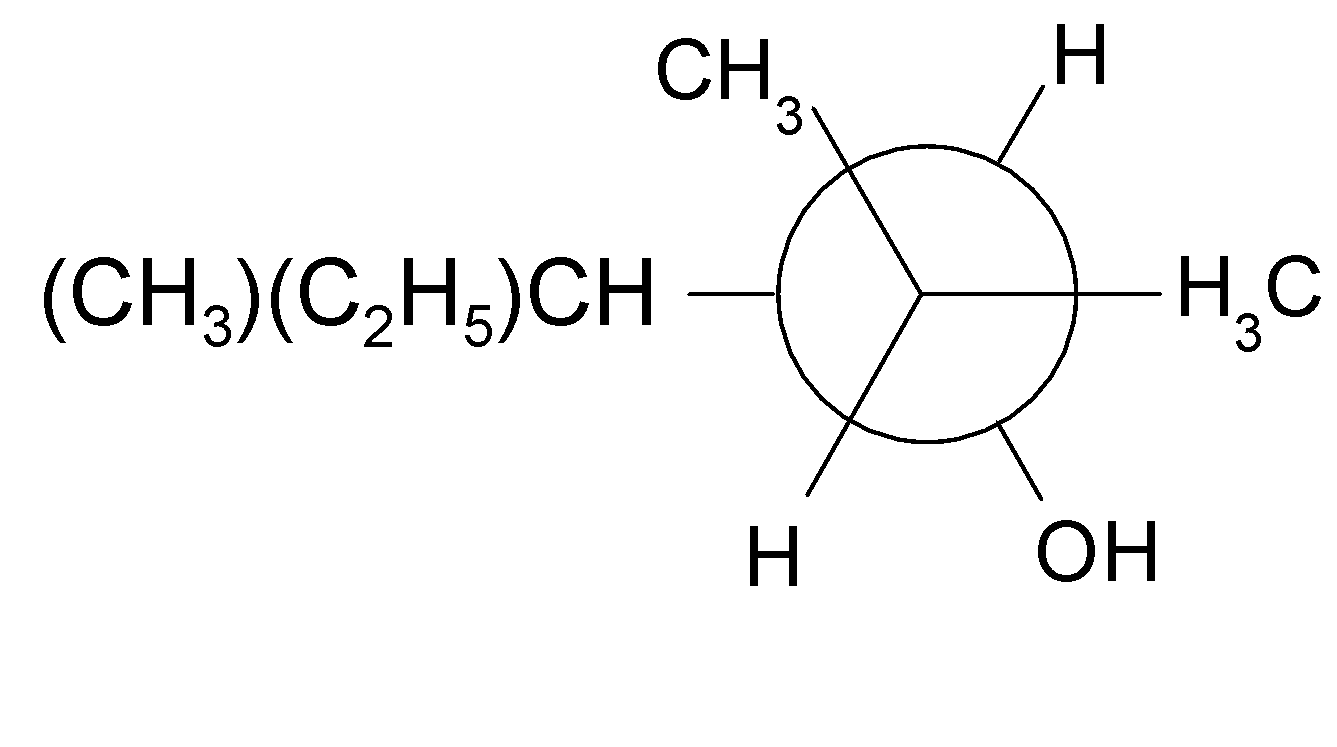
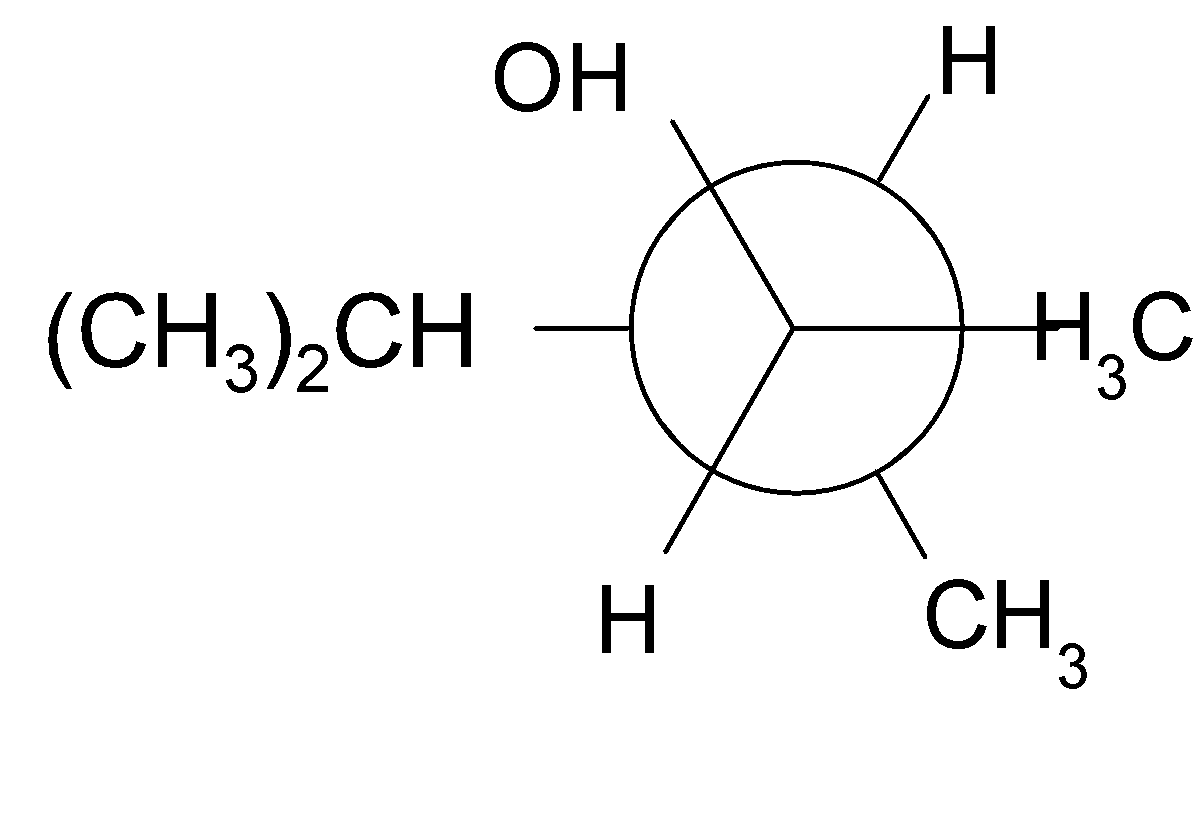
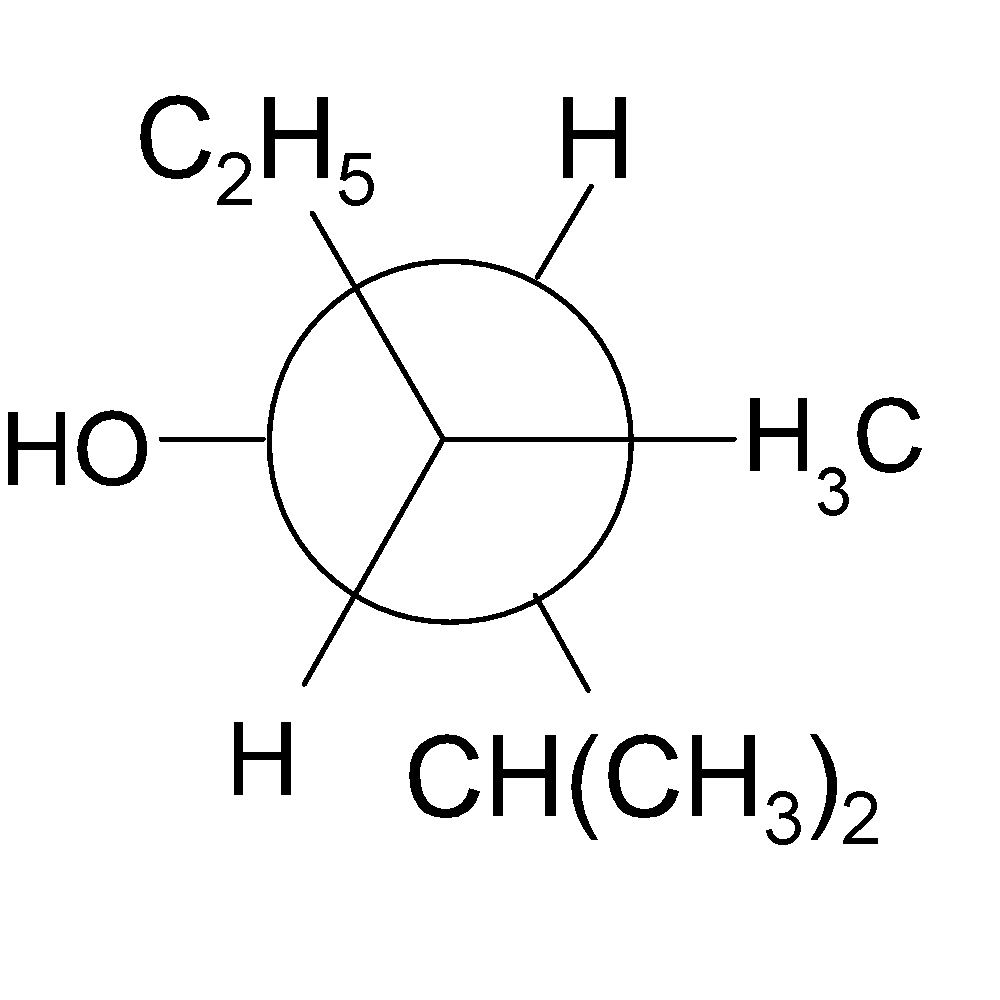
4-3 Each of the following descriptions applies to more than one alkane. In each case, draw and name two structures that match the description.
(a) a sec-butylheptane
(b) a trans-dimethylcyclobutane
(c) a cis-di-tert-butylcyclohexane
(d) an isopropyloctane
(e) a (1,2-dimethylpropyl)cycloalkane
(f) a bicycloheptane
4-4 Write structures for a homologous series of alcohols (R-OH) having from one to five carbons.
4-5 In each pair of compound, which compound has the higher boiling point? Explain your reasoning.
(a) Nonane or 3-ethylhexane
(b) Pentane or 2-methylbutane
(c) Octane or 2,2,4-trimethylpentane
4-6 There are four isomeric four-carbon alkyl groups. Draw them, give their systematic names and label the degree of substitution (primary, secondary, or tertiary) of the head carbon atom which is bonded to the main chain.
4-7 Draw Newman projection of the most stable conformation of the following compounds as viewed from the indicated bond.
(a) 3-methylhexane viewed at C3-C4 bond
(b) 2,2-dimethylbutane viewed at C2-C3 bond
4-8
(a) Draw two chair conformations of trans-1,2-dimethylcyclohexane and label all position as (a) for axial or (e) for equatorial.
(b) Determine the higher-energy and the lower-energy conformations
(c) Calculate the energy difference in these two conformations
4-9 Draw the two chair conformations of each compound and label the substituents as axial or equatorial. In each case, determine which conformation is more stable.
(a) cis-1-ethyl-4-methylcyclohexane
(b) trans-1-ethyl-4-methylcyclohexane
(c) cis-1-bromo-3-methylcyclohexane
(d) trans-1-bromo-3-methylcyclohexane
(e) cis-1-methyl-2-isopropylcychlohexane
(f) trans-1-methyl-2-isopropylchyclohexane
4-10 Glucose with molecular formula C6H12O6 is by far the most abundant sugar in nature. Glucose can take form as an open chain or as can be closed into a ring form. Below are chair conformations of α and β D-glucose. Using what you know about the conformational energy of substituted cyclohexane, predict which of the two isomers predominates in equilibrium. Explain your reasoning.

4-11 Provide a line drawing corresponding to each of the following Newman projections and name them using IUPAC rules.
(a) 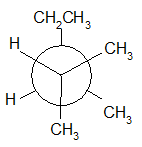
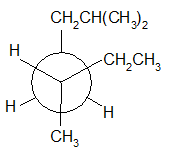
(b)
(c)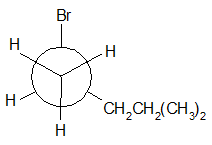
(d) 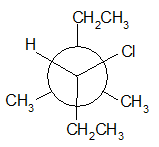
(e) 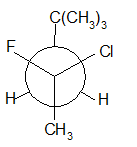
(f)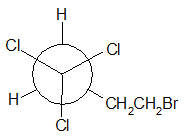
(g) 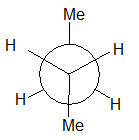
(h) 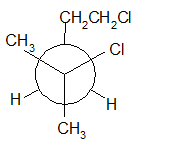
(i)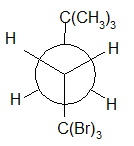
(j)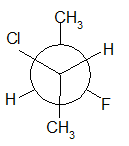
4-12 Draw Newman projections along the C3-C4 bond to show the most stable and the least stable conformation of 2,3,5-trimethylhexane.
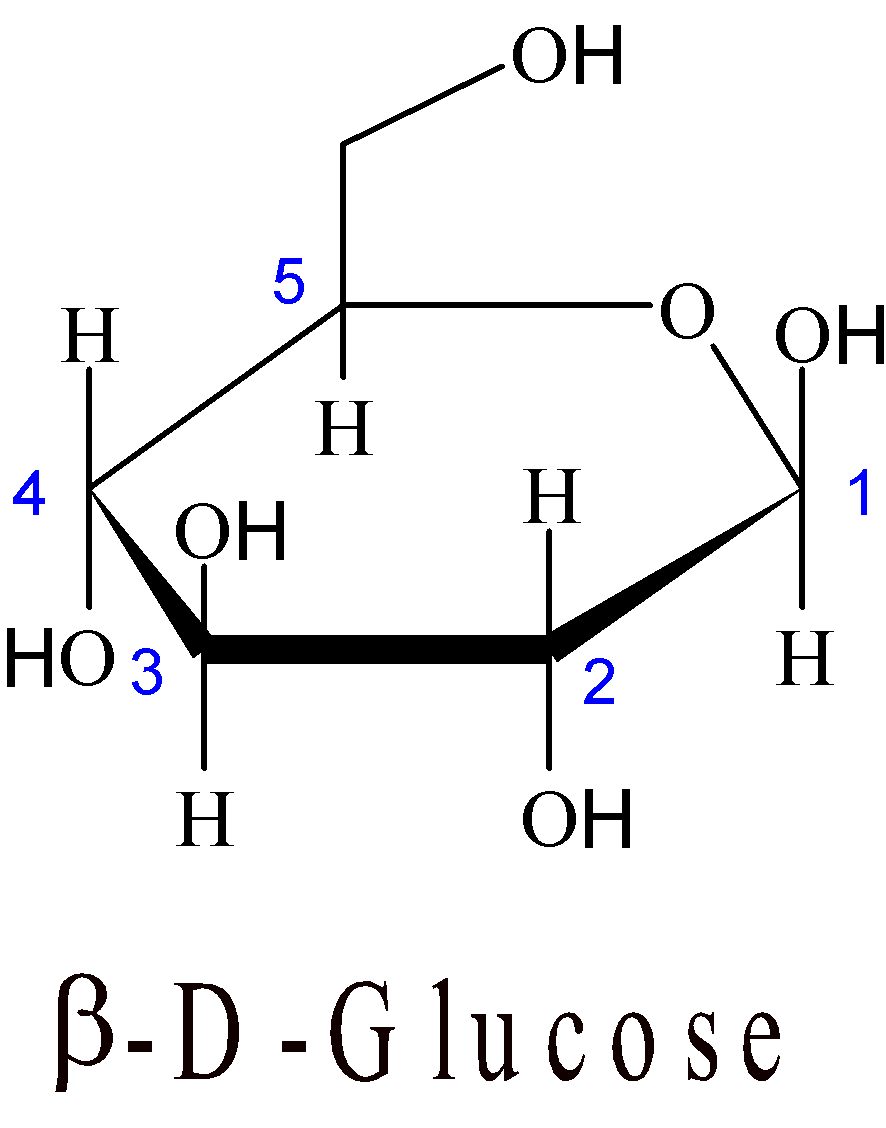
4-13 In β-D-glucose, the hydroxyl group in C1 position is cis to the CH2OH group in C5 position, as shown in the figure below.
There are two chair conformations of β-D-glucose. Draw them and identify which conformation is more stable.