3.1: Half-Life
- Page ID
- 288458
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
Learning Objectives
- To define half-life.
- To solve word problems involving half-life, time, and mass of a radioactive substance.
Whether or not a given isotope is radioactive is a characteristic of that particular isotope. Some isotopes are stable indefinitely, while others are radioactive and decay through a characteristic form of emission. As time passes, less and less of the radioactive isotope will be present, and the level of radioactivity decreases. An interesting and useful aspect of radioactive decay is the half-life. The half-life of a radioactive isotope is the amount of time it takes for one-half of the radioactive isotope to decay. The half-life of a specific radioactive isotope is constant; it is unaffected by conditions and is independent of the initial amount of that isotope.
Consider the following example. Suppose we have 100.0 g of 3H (tritium, a radioactive isotope of hydrogen). It has a half-life of 12.3 y. After 12.3 y, half of the sample will have decayed to 3He by emitting a beta particle, so that only 50.0 g of the original 3H remains. After another 12.3 y—making a total of 24.6 y—another half of the remaining 3H will have decayed, leaving 25.0 g of 3H. After another 12.3 y—now a total of 36.9 y—another half of the remaining 3H will have decayed, leaving 12.5 g of 3H. This sequence of events is illustrated in Figure \(\PageIndex{1}\).
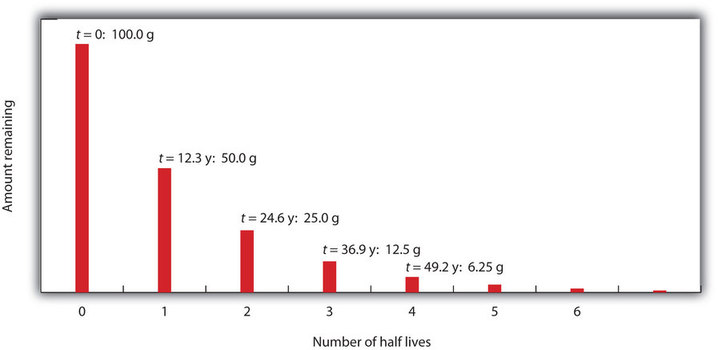
We can determine the amount of a radioactive isotope remaining after a given number half-lives by using the following expression:
\[\mathrm{amount\: remaining=initial\: amount\times \left ( \dfrac{1}{2} \right )^n} \label{Eq1}\]
where n is the number of half-lives. This expression works even if the number of half-lives is not a whole number.
Example \(\PageIndex{1}\): Fluorine-20
The half-life of 20F is 11.0 s. If a sample initially contains 5.00 g of 20F, how much 20F remains after 44.0 s?
Solution
If we compare the time that has passed to the isotope’s half-life, we note that 44.0 s is exactly 4 half-lives, so we can use Equation \ref{Eq1} with \(n = 4\). Substituting and solving results in the following:
\[ \begin{align*} \mathrm{amount\: remaining} &=5.00\: g \times \left(\dfrac{1}{2}\right)^4 \\[4pt] &=5.00\: g \times \dfrac{1}{16} \\[4pt] &=0.313\: g \end{align*}\]
Less than one-third of a gram of 20F remains.
Exercise \(\PageIndex{1}\): Titanium-44
The half-life of 44Ti is 60.0 y. A sample initially contains 0.600 g of 44Ti. How much 44Ti remains after 180.0 y?
- Answer
-
0.075 g.
Half-lives of isotopes range from fractions of a microsecond to billions of years. Table \(\PageIndex{1}\) lists the half-lives of some isotopes.
| Isotope | Half-Life |
|---|---|
| 3H | 12.3 y |
| 14C | 5,730 y |
| 40K | 1.26 × 109 y |
| 51Cr | 27.70 d |
| 90Sr | 29.1 y |
| 131I | 8.04 d |
| 222Rn | 3.823 d |
| 235U | 7.04 × 108 y |
| 238U | 4.47 × 109 y |
| 241Am | 432.7 y |
| 248Bk | 23.7 h |
| 260Sg | 4 ms |
Example \(\PageIndex{2}\): Iodine-131
The isotope \(\ce{I}\)-131 is used in treatment for thyroid cancer and has a half-life of 8.05 days. If a clinic initially has 400 mg, how long will it take for the sample to decay to only 25 mg of \(\ce{I}\)-131?
Solution
We begin by determining how many half-lives it would take for the sample to decay from 400 mg to 25 mg of \(\ce{I}\)-131. There are several ways to do this! We know that for each half-life the mass of \(\ce{I}\)-131 is reduced to one half of the previous value so we could solve this conceptually:
\[ \begin{align*} \dfrac{400 \: \text{mg}}{2} = 200 \: \text{mg} \\ \dfrac{200 \: \text{mg}}{2} = 100 \: \text{mg} \\ \dfrac{100 \: \text{mg}}{2} = 50 \: \text{mg} \\ \dfrac{50 \: \text{mg}}{2} = 25 \: \text{mg} \end{align*}\]
We had to divide the original mass by 2 four times to get the final mass. Therefore, it took four half-lives to get from 400 mg to 25 mg of \(\ce{I}\)-131.
Once the number of half-lives has been determined, we can calculate the total time.
\[ \begin{align*} \text{total time} = \text{half-life}\times \text{number of half-lives} \\ = 8.05 \: \text{days} \times 4 \: \text{half-lives} = 32.2 \: \text{days} \end{align*} \]
A more sophisticated way to solve the problem is to make use of percentages or fractions. The graph below shows the percentage of \(\ce{I}\)-131 remaining after each half-life. These percentages are always the same, regardless of the initial mass of \(\ce{I}\)-131.
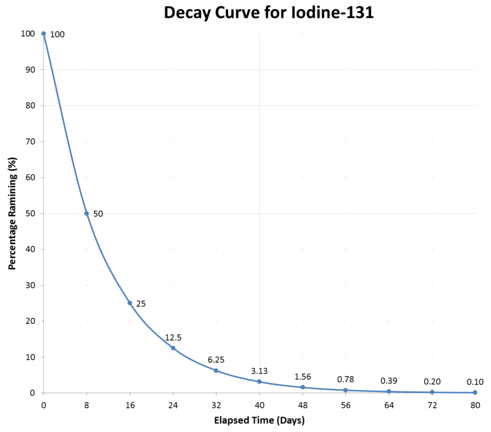
Typical radioactive decay curve.
The graph above illustrates a typical decay curve. Since the half-life for \(\ce{I}-131\) is 8.05 days, the x-axis has units of days. The y-axis indicates the percentage of sample remaining. Initially, (at elapsed time = 0), 100 % of the sample remains. After one half-life (8.05 days for \(\ce{I}-131\)), 50 % remains. After two half-lives, 25 % of the initial sample remains. We can use this pattern as an alternate way of calculating the number of half-lives when the initial and final masses are known.
Alternate method of solving:
\[\dfrac{\text{final mass}}{\text{initial mass}}\times 100 = \text{percentage remaining} \]
\[\dfrac{25 \: \text{mg}}{400 \: \text{mg}}\times 100 = 6.25 \text{%} \nonumber \]
This result, 6.25 % remaining, corresponds to four half-lives. Careful! Don't count 100 % as the first half-life. At time = 0, nothing has happened yet. 50 % remaining = 1 half-life, 25 % remaining = 2 half-lives, 12.5 % remaining = 3 half-lives, and 6.25 % remaining = 4 half-lives. These percentages are the same for all isotopes so you could calculate them once and then use them for multiple problems.
To finish this problem, multiply the number of half-lives by the length of each half-life to get the total time.
Exercise \(\PageIndex{2}\)
A sample of \(\ce{Ac}\)-225 originally contained 80 grams and after 50 days only 2.5 grams of the original \(\ce{Ac}\)-225 remain. What is the half life of \(\ce{Ac}\)-225?
- Answer
-
10 days. Notice that the phrase "what is the half-life?" means "how long is the half-life?".
Looking Closer: Half-Lives of Radioactive Elements
Many people think that the half-life of a radioactive element represents the amount of time an element is radioactive. In fact, it is the time required for half—not all—of the element to decay radioactively. Occasionally, however, the daughter element is also radioactive, so its radioactivity must also be considered.
The expected working life of an ionization-type smoke detector (described in the opening essay) is about 10 years. In that time, americium-241, which has a half-life of about 432 y, loses less than 4% of its radioactivity. A half-life of 432 y may seem long to us, but it is not very long as half-lives go. Uranium-238, the most common isotope of uranium, has a half-life of about 4.5 × 109 y, while thorium-232 has a half-life of 14 × 109 y.
On the other hand, some nuclei have extremely short half-lives, presenting challenges to the scientists who study them. The longest-lived isotope of lawrencium, 262Lr, has a half-life of 3.6 h, while the shortest-lived isotope of lawrencium, 252Lr, has a half-life of 0.36 s. As of this writing, the largest atom ever detected has atomic number 118, mass number 293, and a half-life of 120 ns. Can you imagine how quickly an experiment must be done to determine the properties of elements that exist for so short a time?
Key Takeaways
- Natural radioactive processes are characterized by a half-life, the time it takes for half of the material to decay radioactively.
- The amount of material left over after a certain number of half-lives can be easily calculated.

