12.E: Stoichiometry Applications (Exercises)
- Page ID
- 367877
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)The following questions are related to the material covered in this chapter. For additional discussion on each topic, also check the links included in each heading.
5.1: Stoichiometry
- Think back to the pound cake recipe. What possible conversion factors can you construct relating the components of the recipe?
- Think back to the pancake recipe. What possible conversion factors can you construct relating the components of the recipe?
- What are all the conversion factors that can be constructed from the balanced chemical reaction: \[\ce{2H2(g) + O2(g) → 2H2O(ℓ)}?\]
- What are all the conversion factors that can be constructed from the balanced chemical reaction N2(g) + 3H2(g) → 2NH3(g)?
- Given the chemical equation : Na(s) + H2O(ℓ) → NaOH(aq) + H2(g)
- Balance the equation.
- How many molecules of H2 are produced when 332 atoms of Na react?
- Given the chemical equation: S(s) + O2(g) → SO3(g)
- Balance the equation.
- How many molecules of O2 are needed when 38 atoms of S react?
- For the balanced chemical equation:
6H+(aq) + 2MnO4−(aq) + 5H2O2(ℓ) → 2Mn2+(aq) + 5O2(g) + 8H2O(ℓ)
how many molecules of H2O are produced when 75 molecules of H2O2 react?
- For the balanced chemical reaction 2C6H6(ℓ) + 15O2(g) → 12CO2(g) + 6H2O(ℓ)
how many molecules of CO2 are produced when 56 molecules of C6H6 react?
- Given the balanced chemical equation Fe2O3(s) + 3SO3(g) → Fe2(SO4)3
how many molecules of Fe2(SO4)3 are produced if 321 atoms of S are reacted?
- For the balanced chemical equation CuO(s) + H2S(g) → CuS + H2O(ℓ)
how many molecules of CuS are formed if 9,044 atoms of H react?
- For the balanced chemical equation Fe2O3(s) + 3SO3(g) → Fe2(SO4)3
suppose we need to make 145,000 molecules of Fe2(SO4)3. How many molecules of SO3 do we need?
- One way to make sulfur hexafluoride is to react thioformaldehyde, CH2S, with elemental fluorine:
CH2S + 6F2 → CF4 + 2HF + SF6
If 45,750 molecules of SF6 are needed, how many molecules of F2 are required?
- Construct the three independent conversion factors possible for these two reactions:
- 2H2 + O2 → 2H2O
- H2 + O2 → H2O2
Why are the ratios between H2 and O2 different?
The conversion factors are different because the stoichiometries of the balanced chemical reactions are different.
- Construct the three independent conversion factors possible for these two reactions:
- 2Na + Cl2 → 2NaCl
- 4Na + 2Cl2 → 4NaCl
What similarities, if any, exist in the conversion factors from these two reactions?
- \[\frac{1\, pound\, butter}{1\, pound\, flour}\] or \[\frac{1\, pound\, sugar}{1\, pound\, eggs}\] are two conversion factors that can be constructed from the pound cake recipe. Other conversion factors are also possible.1 pound butter1 pound flour
-
2 molecules H 2 1 molecule O 2 2 molecules H 2 1 molecule O 2 -
-
2Na(s) + 2H2O(ℓ) → 2NaOH(aq) + H2(g)
-
166 molecules
-
-
120 molecules
-
107 molecules
-
435,000 molecules
-
- \[\frac{2\, molecules\, H_{2}}{1\, molecule\, O_{2}}\ , \frac{1\, molecule\, O_{2}}{2\, molecules\, H_{2}O}\ , \frac{2\, molecules\, H_{2}}{2\, molecules\, H_{2}O}\]
- \[\frac{1\, molecules\, H_{2}}{1\, molecule\, O_{2}}\ , \frac{1\, molecule\, O_{2}}{2\, molecules\, H_{2}O_{2}}\ , \frac{1\, molecule\, H_{2}}{1\, molecule\, H_{2}O_{2}}\]
5.4: Mole-Mass and Mass-Mass Calculations
- What mass of CO2 is produced by the combustion of 1.00 mol of CH4?CH4(g) + 2O2(g) → CO2(g) + 2H2O(ℓ)
- What mass of H2O is produced by the combustion of 1.00 mol of CH4? CH4(g) + 2O2(g) → CO2(g) + 2H2O(ℓ)
- What mass of HgO is required to produce 0.692 mol of O2? 2HgO(s) → 2Hg(ℓ) + O2(g)
- What mass of NaHCO3 is needed to produce 2.659 mol of CO2? 2NaHCO3(s) → Na2CO3(s) + H2O(ℓ) + CO2(g)
- How many moles of Al can be produced from 10.87 g of Ag? Al(NO3) 3(s) + 3Ag → Al + 3AgNO3
- How many moles of HCl can be produced from 0.226 g of SOCl2? SOCl2(ℓ) + H2O(ℓ) → SO2(g) + 2HCl(g)
- How many moles of O2 are needed to prepare 1.00 g of Ca(NO3)2? Ca(s) + N2(g) + 3O2(g) → Ca(NO3) 2(s)
- How many moles of C2H5OH are needed to generate 106.7 g of H2O? C2H5OH(ℓ) + 3O2(g) → 2CO2(g) + 3H2O(ℓ)
- What mass of O2 can be generated by the decomposition of 100.0 g of NaClO3? 2NaClO3 → 2NaCl(s) + 3O2(g)
- What mass of Li2O is needed to react with 1,060 g of CO2? Li2O(aq) + CO2(g) → Li2CO3(aq)
- What mass of Fe2O3 must be reacted to generate 324 g of Al2O3? Fe2O3(s) + 2Al(s) → 2Fe(s) + Al2O3(s)
- What mass of Fe is generated when 100.0 g of Al are reacted? Fe2O3(s) + 2Al(s) → 2Fe(s) + Al2O3(s)
- What mass of MnO2 is produced when 445 g of H2O are reacted? H2O(ℓ) + 2MnO4−(aq) + Br−(aq) → BrO3−(aq) + 2MnO2(s) + 2OH−(aq)
- What mass of PbSO4 is produced when 29.6 g of H2SO4 are reacted? Pb(s) + PbO2(s) + 2H2SO4(aq) → 2PbSO4(s) + 2H2O(ℓ)
- If 83.9 g of ZnO are formed, what mass of Mn2O3 is formed with it? Zn(s) + 2MnO2(s) → ZnO(s) + Mn2O3(s)
- If 14.7 g of NO2 are reacted, what mass of H2O is reacted with it? 3NO2(g) + H2O(ℓ) → 2HNO3(aq) + NO(g)
- If 88.4 g of CH2S are reacted, what mass of HF is produced? CH2S + 6F2 → CF4 + 2HF + SF6
- If 100.0 g of Cl2 are needed, what mass of NaOCl must be reacted?
NaOCl + HCl → NaOH + Cl2
Answers
- 44.0 g
-
3.00 × 102 g
-
0.0336 mol
-
0.0183 mol
-
45.1 g
-
507 g
-
4.30 × 103 g
-
163 g
-
76.7 g
5.5: Yields
- What is the difference between the theoretical yield and the actual yield?
- What is the difference between the actual yield and the percent yield?
- A worker isolates 2.675 g of SiF4 after reacting 2.339 g of SiO2 with HF. What are the theoretical yield and the actual yield? SiO2(s) + 4HF(g) → SiF4(g) + 2H2O(ℓ)
- A worker synthesizes aspirin, C9H8O4, according to this chemical equation. If 12.66 g of C7H6O3 are reacted and 12.03 g of aspirin are isolated, what are the theoretical yield and the actual yield? C7H6O3 + C4H6O3 → C9H8O4 + HC2H3O2
- A chemist decomposes 1.006 g of NaHCO3 and obtains 0.0334 g of Na2CO3. What are the theoretical yield and the actual yield? 2NaHCO3(s) → Na2CO3(s) + H2O(ℓ) + CO2(g)
- A chemist combusts a 3.009 g sample of C5H12 and obtains 3.774 g of H2O. What are the theoretical yield and the actual yield? C5H12(ℓ) + 8O2(g) → 5CO2 + 6H2O(ℓ)
- What is the percent yield in Exercise 3?
- What is the percent yield in Exercise 4?
- What is the percent yield in Exercise 5?
- What is the percent yield in Exercise 6?
Answers
- Theoretical yield is what you expect stoichiometrically from a chemical reaction; actual yield is what you actually get from a chemical reaction.
-
theoretical yield = 4.052 g; actual yield = 2.675 g
-
theoretical yield = 0.635 g; actual yield = 0.0334 g
-
66.02%
-
5.26%
5.6: Limiting Reagents
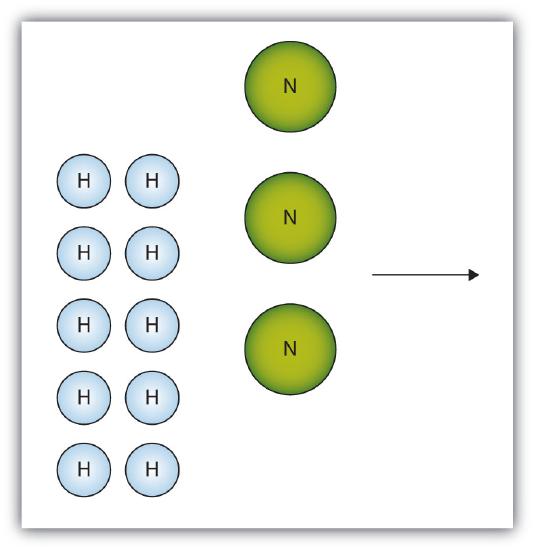
- The box below shows a group of nitrogen and hydrogen molecules that will react to produce ammonia, NH3. What is the limiting reagent?
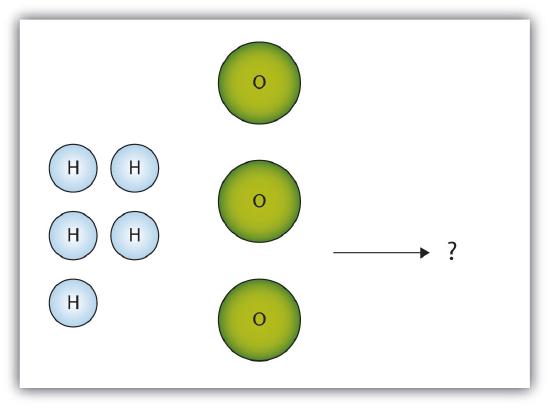
- The box below shows a group of hydrogen and oxygen molecules that will react to produce water, H2O. What is the limiting reagent?
- Given the statement “20.0 g of methane is burned in excess oxygen,” is it obvious which reactant is the limiting reagent?
- Given the statement “the metal is heated in the presence of excess hydrogen,” is it obvious which substance is the limiting reagent despite not specifying any quantity of reactant?
- Acetylene (C2H2) is formed by reacting 7.08 g of C and 4.92 g of H2. 2C(s) + H2(g) → C2H2(g)
What is the limiting reagent? How much of the other reactant is in excess?
- Ethane (C2H6) is formed by reacting 7.08 g of C and 4.92 g of H2. 2C(s) + 3H2(g) → C2H6(g)
What is the limiting reagent? How much of the other reactant is in excess?
- Given the initial amounts listed, what is the limiting reagent, and how much of the other reactant is in excess?
\[\underset{35.6\, g}{P_{4}O_{6}(s)}+6\underset{4.77\, g}{H_{2}O(l)}\rightarrow 4H_{3}PO_{4}\]
- Given the initial amounts listed, what is the limiting reagent, and how much of the other reactant is in excess?
\[\underset{377\, g}{3NO_{2}(g)}+\underset{244\, g}{H_{2}O(l)}\rightarrow 2HNO_{3}(aq)+NO(g)\]
- To form the precipitate PbCl2, 2.88 g of NaCl and 7.21 g of Pb(NO3)2 are mixed in solution. How much precipitate is formed? How much of which reactant is in excess?
- In a neutralization reaction, 18.06 g of KOH are reacted with 13.43 g of HNO3. What mass of H2O is produced, and what mass of which reactant is in excess?
Answers
- Nitrogen is the limiting reagent.
-
Yes; methane is the limiting reagent.
-
C is the limiting reagent; 4.33 g of H2 are left over.
-
H2O is the limiting reagent; 25.9 g of P4O6 are left over.
-
6.06 g of PbCl2 are formed; 0.33 g of NaCl is left over.
7 Energy and Chemical Processes
1. Sulfur dioxide (SO2) is a pollutant gas that is one cause of acid rain. It is oxidized in the atmosphere to sulfur trioxide (SO3), which then combines with water to make sulfuric acid (H2SO4).
- Write the balanced reaction for the oxidation of SO2 to make SO3. (The other reactant is diatomic oxygen.)
- When 1 mol of SO2 reacts to make SO3, 23.6 kcal of energy are given off. If 100 lb (1 lb = 454 g) of SO2 were converted to SO3, what would be the total energy change?
2. Ammonia (NH3) is made by the direct combination of H2 and N2 gases according to this reaction:
N2(g) + 3H2(g) → 2NH3(g) + 22.0 kcal
- Is this reaction endothermic or exothermic?
- What is the overall energy change if 1,500 g of N2 are reacted to make ammonia?
3. A 5.69 g sample of iron metal was heated in boiling water to 99.8°C. Then it was dropped into a beaker containing 100.0 g of H2O at 22.6°C. Assuming that the water gained all the heat lost by the iron, what is the final temperature of the H2O and Fe?
4. A 5.69 g sample of copper metal was heated in boiling water to 99.8°C. Then it was dropped into a beaker containing 100.0 g of H2O at 22.6°C. Assuming that the water gained all the heat lost by the copper, what is the final temperature of the H2O and Cu?
5. When 1 g of steam condenses, 540 cal of energy is released. How many grams of ice can be melted with 540 cal?
6. When 1 g of water freezes, 79.9 cal of energy is released. How many grams of water can be boiled with 79.9 cal?
7. The change in energy is +65.3 kJ for each mole of calcium hydroxide [Ca(OH)2] according to the following reaction:
Ca(OH)2(s) → CaO(s) + H2O(g)
8. How many grams of Ca(OH)2 could be reacted if 575 kJ of energy were available?
9. The thermite reaction gives off so much energy that the elemental iron formed as a product is typically produced in the liquid state:
2Al(s) + Fe2O3(s) → Al2O3(s) + 2Fe(ℓ) + 204 kcal
How much heat will be given off if 250 g of Fe are to be produced?
10. A normal adult male requires 2,500 kcal per day to maintain his metabolism.
- Nutritionists recommend that no more than 30% of the calories in a person’s diet come from fat. At 9 kcal/g, what is the maximum mass of fat an adult male should consume daily?
- At 4 kcal/g each, how many grams of protein and carbohydrates should an adult male consume daily?
11. A normal adult male requires 2,500 kcal per day to maintain his metabolism.
- At 9 kcal/g, what mass of fat would provide that many kilocalories if the diet was composed of nothing but fats?
- At 4 kcal/g each, what mass of protein and/or carbohydrates is needed to provide that many kilocalories?
12. The volume of the world’s oceans is approximately 1.34 × 1024 cm3.
- How much energy would be needed to increase the temperature of the world’s oceans by 1°C? Assume that the heat capacity of the oceans is the same as pure water.
- If Earth receives 6.0 × 1022 J of energy per day from the sun, how many days would it take to warm the oceans by 1°C, assuming all the energy went into warming the water?
13. Does a substance that has a small specific heat require a small or large amount of energy to change temperature? Explain.
14. Some biology textbooks represent the conversion of adenosine triphosphate (ATP) to adenosine diphosphate (ADP) and phosphate ions as follows:
ATP → ADP + phosphate + energy
What is wrong with this reaction?
15. Assuming that energy changes are additive, how much energy is required to change 15.0 g of ice at −15°C to 15.0 g of steam at 115°C? (Hint: you will have five processes to consider.)
Answers
- 2SO2 + O2 → 2SO3
- 16,700 kcal
2.
exothermic 1177 kcalabout 23.1°C
4. about 23.0°C
5. 6.76 g
6. 0.148 g
652 g
8. 457 kcal
- 83.3 g
- 438 g
10.
a. 278 g
b. 625 g
11.
1.34 × 1024 cal 93 days12. A substance with smaller specific heat requires less energy per unit of mass to raise its temperature,
13. A reactant is missing: H2O is missing.
14. Total energy = 11,019 cal
5.7: Additional Exercises
- How many molecules of O2 will react with 6.022 × 1023 molecules of H2 to make water? The reaction is 2H2(g) + O2(g) → 2H2O(ℓ).
- How many molecules of H2 will react with 6.022 × 1023 molecules of N2 to make ammonia? The reaction is N2(g) + 3H2(g) → 2NH3(g).
- How many moles are present in 6.411 kg of CO2? How many molecules is this?
- How many moles are present in 2.998 mg of SCl4? How many molecules is this?
- What is the mass in milligrams of 7.22 × 1020 molecules of CO2?
- What is the mass in kilograms of 3.408 × 1025 molecules of SiS2?
- What is the mass in grams of 1 molecule of H2O?
- What is the mass in grams of 1 atom of Al?
- What is the volume of 3.44 mol of Ga if the density of Ga is 6.08 g/mL?
- What is the volume of 0.662 mol of He if the density of He is 0.1785 g/L?
- For the chemical reaction 2C4H10(g) + 13O2(g) → 8CO2(g) + 10H2O(ℓ)
assume that 13.4 g of C4H10 reacts completely to products. The density of CO2 is 1.96 g/L. What volume in liters of CO2 is produced?
- For the chemical reaction 2GaCl3(s) + 3H2(g) → 2Ga(ℓ) + 6HCl(g)
if 223 g of GaCl3 reacts completely to products and the density of Ga is 6.08 g/mL, what volume in milliliters of Ga is produced?
- Calculate the mass of each product when 100.0 g of CuCl react according to the reaction 2CuCl(aq) → CuCl2(aq) + Cu(s)
What do you notice about the sum of the masses of the products? What concept is being illustrated here?
- Calculate the mass of each product when 500.0 g of SnCl2 react according to the reaction 2SnCl2(aq) → SnCl4(aq) + Sn(s)
What do you notice about the sum of the masses of the products? What concept is being illustrated here?
- What mass of CO2 is produced from the combustion of 1 gal of gasoline? The chemical formula of gasoline can be approximated as C8H18. Assume that there are 2,801 g of gasoline per gallon.
- What mass of H2O is produced from the combustion of 1 gal of gasoline? The chemical formula of gasoline can be approximated as C8H18. Assume that there are 2,801 g of gasoline per gallon.
- A chemical reaction has a theoretical yield of 19.98 g and a percent yield of 88.40%. What is the actual yield?
- A chemical reaction has an actual yield of 19.98 g and a percent yield of 88.40%. What is the theoretical yield?
- Given the initial amounts listed, what is the limiting reagent, and how much of the other reactants are in excess?
\[\underset{35.0\, g}{P_{4}}+\underset{12.7\, g}{3NaOH}+\underset{9.33\, g}{3H_{2}O}\rightarrow 2Na_{2}HPO_{4}+PH_{3}\]
- Given the initial amounts listed, what is the limiting reagent, and how much of the other reactants are in excess?
\[\underset{46.3\, g}{2NaCrO_{2}}+\underset{88.2\, g}{3NaBrO_{4}}+\underset{32.5\, g}{2NaOH}\rightarrow 3NaBrO_{3}+2Na_{2}CrO_{4}+H_{2}O\]
- Verify that it does not matter which product you use to predict the limiting reagent by using both products in this combustion reaction to determine the limiting reagent and the amount of the reactant in excess. Initial amounts of each reactant are given.
\[\underset{26.3\, g}{C_{3}H_{8}}+\underset{21.8\, g}{5O_{2}}\rightarrow 3CO_{2}(g)+4H_{2}O(l)\]
- Just in case you suspect Exercise 21 is rigged, do it for another chemical reaction and verify that it does not matter which product you use to predict the limiting reagent by using both products in this combustion reaction to determine the limiting reagent and the amount of the reactant in excess. Initial amounts of each reactant are given.
\[\underset{35.0\, g}{2P_{4}}+\underset{12.7\, g}{6NaOH}+\underset{9.33\, g}{6H_{2}O}\rightarrow 3Na_{2}HPO_{4}+5PH_{3}\]
Answers
- 1.2044 × 1024 molecules
-
145.7 mol; 8.77 × 1025 molecules
-
52.8 mg
-
2.99 × 10−23 g
-
39.4 mL
-
20.7 L
-
67.91 g of CuCl2; 32.09 g of Cu. The two masses add to 100.0 g, the initial amount of starting material, demonstrating the law of conservation of matter.
-
8,632 g
-
17.66 g
-
The limiting reagent is NaOH; 21.9 g of P4 and 3.61 g of H2O are left over.
-
Both products predict that O2 is the limiting reagent; 20.3 g of C3H8 are left over.