9.E: Attractive Forces
- Page ID
- 367841
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)The following questions are related to the material covered in this chapter. For additional discussion on each topic, also check the links included in each heading.
10.2: Intermolecular Forces
- What type of intermolecular force do all substances have?
- What is necessary for a molecule to experience dipole-dipole interactions?
- What is necessary for a molecule to experience hydrogen bonding?
- How does varying the temperature change the preferred phase of a substance?
- Identify the strongest intermolecular force present in each substance.
- He
- CHCl3
- HOF
- Identify the strongest intermolecular force present in each substance.
- CH3OH
- (CH3)2CO
- N2
- Identify the strongest intermolecular force present in each substance.
- HBr
- C6H5NH2
- CH4
- Identify the strongest intermolecular force present in each substance.
- C10H22
- HF
- glucose
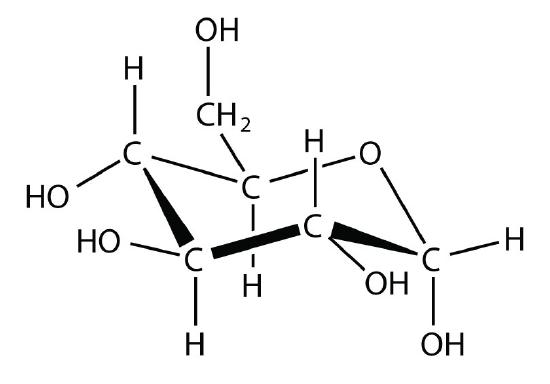
- dispersion force
- An H atom must be bonded to an N, O, or F atom.
-
- dispersion forces
- dipole-dipole interactions
- hydrogen bonding
-
- dipole-dipole interactions
- hydrogen bonding
- dispersion forces
10.3: Phase Transitions - Melting, Boiling, and Subliming
- What is the difference between melting and solidification?
- What is the difference between boiling and condensation?
- Describe the molecular changes when a solid becomes a liquid.
- Describe the molecular changes when a liquid becomes a gas.
- What is the energy change when 78.0 g of Hg melt at −38.8°C?
- What is the energy change when 30.8 g of Al solidify at 660°C?
- What is the energy change when 111 g of Br2 boil at 59.5°C?
- What is the energy change when 98.6 g of H2O condense at 100°C?
- Each of the following statements is incorrect. Rewrite them so they are correct.
- Temperature changes during a phase change.
- The process of a liquid becoming a gas is called sublimation.
- Each of the following statements is incorrect. Rewrite them so they are correct.
- The volume of a gas contains only about 10% matter, with the rest being empty space.
- ΔHsub is equal to ΔHvap.
- Write the chemical equation for the melting of elemental sodium.
- Write the chemical equation for the solidification of benzene (C6H6).
- Write the chemical equation for the sublimation of CO2.
- Write the chemical equation for the boiling of propanol (C3H7OH).
- What is the ΔHsub of H2O? (Hint: see Table 10.3.1 "Enthalpies of Fusion for Various Substances" and Table 10.3.2 "Enthalpies of Vaporization for Various Substances".)
- The ΔHsub of I2 is 60.46 kJ/mol, while its ΔHvap is 41.71 kJ/mol. What is the ΔHfus of I2?
Answers
- Melting is the phase change from a solid to a liquid, whereas solidification is the phase change from a liquid to a solid.
- The molecules have enough energy to move about each other but not enough to completely separate from each other.
- 890 J
- 10.7 kJ
-
- Temperature does not change during a phase change.
- The process of a liquid becoming a gas is called boiling; the process of a solid becoming a gas is called sublimation.
- Na(s) → Na(ℓ)
- CO2(s) → CO2(g)
- 46.69 kJ/mol
10.4: Properties of Liquids
- What is the difference between evaporation and boiling?
- What is the difference between a gas and vapor?
- Define normal boiling point in terms of vapor pressure.
- Is the boiling point higher or lower at higher environmental pressures? Explain your answer.
Answers
- Evaporation occurs when a liquid becomes a gas at temperatures below that liquid’s boiling point, whereas boiling is the conversion of a liquid to a gas at the liquid’s boiling point.
-
the temperature at which the vapor pressure of a liquid is 760 torr
10.5: Solids
- What is the difference between a crystalline solid and an amorphous solid?
- What two properties do solids have in common? What two properties of solids can vary?
- Explain how the bonding in an ionic solid explains some of the properties of these solids.
- Explain how the bonding in a molecular solid explains some of the properties of these solids.
- Explain how the bonding in a covalent network solid explains some of the properties of these solids.
- Explain how the bonding in a metallic solid explains some of the properties of these solids.
- Which type(s) of solid has/have high melting points?
- Which type(s) of solid conduct(s) electricity in their solid state? In their liquid state?
- Which type of solid(s) is/are considered relatively soft?
- Which type of solid(s) is/are considered very hard?
- Predict the type of solid exhibited by each substance.
- Hg
- PH3
- CaF2
- Predict the type of solid exhibited by each substance.
- (CH2)n (polyethylene, a form of plastic)
- PCl3
- NH4Cl
- Predict the type of solid exhibited by each substance.
- SO3
- Br2
- Na2SO3
- Predict the type of solid exhibited by each substance.
- BN (boron nitride, a diamond-like compound)
- B2O3
- NaBF4
- Predict the type of solid exhibited by each substance.
- H2S
- Si
- CsF
- Predict the type of solid exhibited by each substance.
- Co
- CO
- CaCO3
Answers
- At the atomic level, a crystalline solid has a regular arrangement of atoms, whereas an amorphous solid has a random arrangement of atoms.
- The oppositely charged ions are very strongly held together, so ionic crystals have high melting points. Ionic crystals are also brittle because any distortion of the crystal moves same-charged ions closer to each other, so they repel.
- The covalent network solid is essentially one molecule, making it very hard and giving it a very high melting point.
- ionic solids, covalent network solids
- molecular solids
-
- metallic
- molecular solid
- ionic crystal
-
- molecular solid
- molecular solid
- ionic crystal
-
- molecular solid
- molecular solid
- ionic crystal
4.1: General Properties of Aqueous Solutions
- What are the advantages to carrying out a reaction in solution rather than simply mixing the pure reactants?
- What types of compounds dissolve in polar solvents?
- Describe the charge distribution in liquid water. How does this distribution affect its physical properties?
- Must a molecule have an asymmetric charge distribution to be polar? Explain your answer.
- Why are many ionic substances soluble in water?
- Explain the phrase like dissolves like.
- What kinds of covalent compounds are soluble in water?
- Why do most aromatic hydrocarbons have only limited solubility in water? Would you expect their solubility to be higher, lower, or the same in ethanol compared with water? Why?
- Predict whether each compound will dissolve in water and explain why.
- toluene
- acetic acid
- sodium acetate
- butanol
- pentanoic acid
- Predict whether each compound will dissolve in water and explain why.
- ammonium chloride
- 2-propanol
- heptane
- potassium dichromate
- 2-octanol
- Given water and toluene, predict which is the better solvent for each compound and explain your reasoning.
- sodium cyanide
- benzene
- acetic acid
- sodium ethoxide (CH3CH2ONa)
- Of water and toluene, predict which is the better solvent for each compound and explain your reasoning.
- t-butanol
- calcium chloride
- sucrose
- cyclohexene
- Compound A is divided into three equal samples. The first sample does not dissolve in water, the second sample dissolves only slightly in ethanol, and the third sample dissolves completely in toluene. What does this suggest about the polarity of A?
- You are given a mixture of three solid compounds—A, B, and C—and are told that A is a polar compound, B is slightly polar, and C is nonpolar. Suggest a method for separating these three compounds.
- A laboratory technician is given a sample that contains only sodium chloride, sucrose, and cyclodecanone (a ketone). You must tell the technician how to separate these three compounds from the mixture. What would you suggest?
- Many over-the-counter drugs are sold as ethanol/water solutions rather than as purely aqueous solutions. Give a plausible reason for this practice.
- What distinguishes a weak electrolyte from a strong electrolyte?
- Which organic groups result in aqueous solutions that conduct electricity?
- It is considered highly dangerous to splash barefoot in puddles during a lightning storm. Why?
- Which solution(s) would you expect to conduct electricity well? Explain your reasoning.
- an aqueous solution of sodium chloride
- a solution of ethanol in water
- a solution of calcium chloride in water
- a solution of sucrose in water
- Which solution(s) would you expect to conduct electricity well? Explain your reasoning.
- an aqueous solution of acetic acid
- an aqueous solution of potassium hydroxide
- a solution of ethylene glycol in water
- a solution of ammonium chloride in water
- Which of the following is a strong electrolyte, a weak electrolyte, or a nonelectrolyte in an aqueous solution? Explain your reasoning.
- potassium hydroxide
- ammonia
- calcium chloride
- butanoic acid
- Which of the following is a strong electrolyte, a weak electrolyte, or a nonelectrolyte in an aqueous solution? Explain your reasoning.
- magnesium hydroxide
- butanol
- ammonium bromide
- pentanoic acid
- Which of the following is a strong electrolyte, a weak electrolyte, or a nonelectrolyte in aqueous solution? Explain your reasoning.
- H2SO4
- diethylamine
- 2-propanol
- ammonium chloride
- propanoic acid
Conceptual Answers
- Ionic compounds such as NaCl are held together by electrostatic interactions between oppositely charged ions in the highly ordered solid. When an ionic compound dissolves in water, the partially negatively charged oxygen atoms of the H2O molecules surround the cations, and the partially positively charged hydrogen atoms in H2O surround the anions. The favorable electrostatic interactions between water and the ions compensate for the loss of the electrostatic interactions between ions in the solid.
-
- Because toluene is an aromatic hydrocarbon that lacks polar groups, it is unlikely to form a homogenous solution in water.
- Acetic acid contains a carboxylic acid group attached to a small alkyl group (a methyl group). Consequently, the polar characteristics of the carboxylic acid group will be dominant, and acetic acid will form a homogenous solution with water.
- Because most sodium salts are soluble, sodium acetate should form a homogenous solution with water.
- Like all alcohols, butanol contains an −OH group that can interact well with water. The alkyl group is rather large, consisting of a 4-carbon chain. In this case, the nonpolar character of the alkyl group is likely to be as important as the polar character of the –OH, decreasing the likelihood that butanol will form a homogeneous solution with water.
- Like acetic acid, pentanoic acid is a carboxylic acid. Unlike acetic acid, however, the alkyl group is rather large, consisting of a 4-carbon chain as in butanol. As with butanol, the nonpolar character of the alkyl group is likely to be as important as the polar character of the carboxylic acid group, making it unlikely that pentanoic acid will form a homogeneous solution with water. (In fact, the solubility of both butanol and pentanoic acid in water is quite low, only about 3 g per 100 g water at 25°C.)
- An electrolyte is any compound that can form ions when it dissolves in water. When a strong electrolyte dissolves in water, it dissociates completely to give the constituent ions. In contrast, when a weak electrolyte dissolves in water, it produces relatively few ions in solution.
4.2: Types of Chemical Reactions - Single and Double Displacement Reactions
- Assuming that each double-replacement reaction occurs, predict the products and write each balanced chemical equation.
- Zn(NO3)2 + NaOH → ?
- HCl + Na2S → ?
- Assuming that each double-replacement reaction occurs, predict the products and write each balanced chemical equation.
- Ca(C2H3O2)2 + HNO3 → ?
- Na2CO3 + Sr(NO2)2 → ?
- Assuming that each double-replacement reaction occurs, predict the products and write each balanced chemical equation.
- Pb(NO3)2 + KBr → ?
- K2O + MgCO3 → ?
- Assuming that each double-replacement reaction occurs, predict the products and write each balanced chemical equation.
- Sn(OH)2 + FeBr3 → ?
- CsNO3 + KCl → ?
- Use the solubility rules to predict if each double-replacement reaction will occur and, if so, write a balanced chemical equation.
- Pb(NO3)2 + KBr → ?
- K2O + Na2CO3 → ?
- Use the solubility rules to predict if each double-replacement reaction will occur and, if so, write a balanced chemical equation.
- Na2CO3 + Sr(NO2)2 → ?
- (NH4)2SO4 + Ba(NO3)2 → ?
- Use the solubility rules to predict if each double-replacement reaction will occur and, if so, write a balanced chemical equation.
- K3PO4 + SrCl2 → ?
- NaOH + MgCl2 → ?
- Use the solubility rules to predict if each double-replacement reaction will occur and, if so, write a balanced chemical equation.
- KC2H3O2 + Li2CO3 → ?
- KOH + AgNO3 → ?
Answers
1. Zn(NO3)2 + 2NaOH → Zn(OH)2 + 2NaNO3- 2HCl + Na2S → 2NaCl + H2S
-
- Pb(NO3)2 + 2KBr → PbBr2 + 2KNO3
- K2O + MgCO3 → K2CO3 + MgO
-
- Pb(NO3)2 + 2KBr → PbBr2(s) + 2KNO3
- No reaction occurs.
-
- 2K3PO4 + 3SrCl2 → Sr3(PO4)2(s) + 6KCl
- 2NaOH + MgCl2 → 2NaCl + Mg(OH)2(s)
4.3: Ionic Equations - A Closer Look
- Write a chemical equation that represents NaBr(s) dissociating in water.
- Write a chemical equation that represents SrCl2(s) dissociating in water.
- Write a chemical equation that represents (NH4)3PO4(s) dissociating in water.
- Write a chemical equation that represents Fe(C2H3O2)3(s) dissociating in water.
- Write the complete ionic equation for the reaction of FeCl2(aq) and AgNO3(aq). You may have to consult the solubility rules.
- Write the complete ionic equation for the reaction of BaCl2(aq) and Na2SO4(aq). You may have to consult the solubility rules.
- Write the complete ionic equation for the reaction of KCl(aq) and NaC2H3O2(aq). You may have to consult the solubility rules.
- Write the complete ionic equation for the reaction of Fe2(SO4)3(aq) and Sr(NO3)2(aq). You may have to consult the solubility rules.
- Write the net ionic equation for the reaction of FeCl2(aq) and AgNO3(aq). You may have to consult the solubility rules.
- Write the net ionic equation for the reaction of BaCl2(aq) and Na2SO4(aq). You may have to consult the solubility rules.
- Write the net ionic equation for the reaction of KCl(aq) and NaC2H3O2(aq). You may have to consult the solubility rules.
- Write the net ionic equation for the reaction of Fe2(SO4)3(aq) and Sr(NO3)2(aq). You may have to consult the solubility rules.
- Identify the spectator ions in Exercises 9 and 10.
- Identify the spectator ions in Exercises 11 and 12.
Answers
- NaBr(s) −→H2O−→H2O Na+(aq) + Br−(aq)
- (NH4)3PO4(s) −→H2O−→H2O 3NH4+(aq) + PO43−(aq)
- Fe2+(aq) + 2Cl−(aq) + 2Ag+(aq) + 2NO3−(aq) → Fe2+(aq) + 2NO3−(aq) + 2AgCl(s)
- K+(aq) + Cl−(aq) + Na+(aq) + C2H3O2−(aq) → Na+(aq) + Cl−(aq) + K+(aq) + C2H3O2−(aq)
- 2Cl−(aq) + 2Ag+(aq) → 2AgCl(s)
- There is no overall reaction.
- In Exercise 9, Fe2+(aq) and NO3−(aq) are spectator ions; in Exercise 10, Na+(aq) and Cl−(aq) are spectator ions.


