4.1: General Properties of Aqueous Solutions
- Page ID
- 21714
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- To understand how and why solutions form
The solvent in aqueous solutions is water, which makes up about 70% of the mass of the human body and is essential for life. Many of the chemical reactions that keep us alive depend on the interaction of water molecules with dissolved compounds. Moreover, the presence of large amounts of water on Earth’s surface helps maintain its surface temperature in a range suitable for life. In this section, we describe some of the interactions of water with various substances and introduce you to the characteristics of aqueous solutions.
Polar Substances
As shown in Figure \(\PageIndex{1}\), the individual water molecule consists of two hydrogen atoms bonded to an oxygen atom in a bent (V-shaped) structure. As is typical of group 16 elements, the oxygen atom in each O–H covalent bond attracts electrons more strongly than the hydrogen atom does. Consequently, the oxygen and hydrogen nuclei do not equally share electrons. Instead, hydrogen atoms are electron poor compared with a neutral hydrogen atom and have a partial positive charge, which is indicated by δ+. The oxygen atom, in contrast, is more electron rich than a neutral oxygen atom, so it has a partial negative charge. This charge must be twice as large as the partial positive charge on each hydrogen for the molecule to have a net charge of zero. Thus its charge is indicated by 2δ−. This unequal distribution of charge creates a polar bond in which one portion of the molecule carries a partial negative charge, while the other portion carries a partial positive charge (Figure \(\PageIndex{1}\)). Because of the arrangement of polar bonds in a water molecule, water is described as a polar substance.
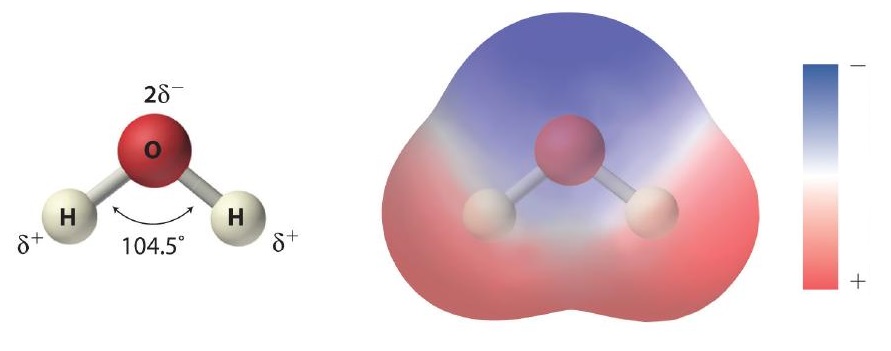
Because of the asymmetric charge distribution in the water molecule, adjacent water molecules are held together by attractive electrostatic (δ+…δ−) interactions between the partially negatively charged oxygen atom of one molecule and the partially positively charged hydrogen atoms of adjacent molecules (Figure \(\PageIndex{2}\)). Energy is needed to overcome these electrostatic attractions. In fact, without them, water would evaporate at a much lower temperature, and neither Earth’s oceans nor we would exist!
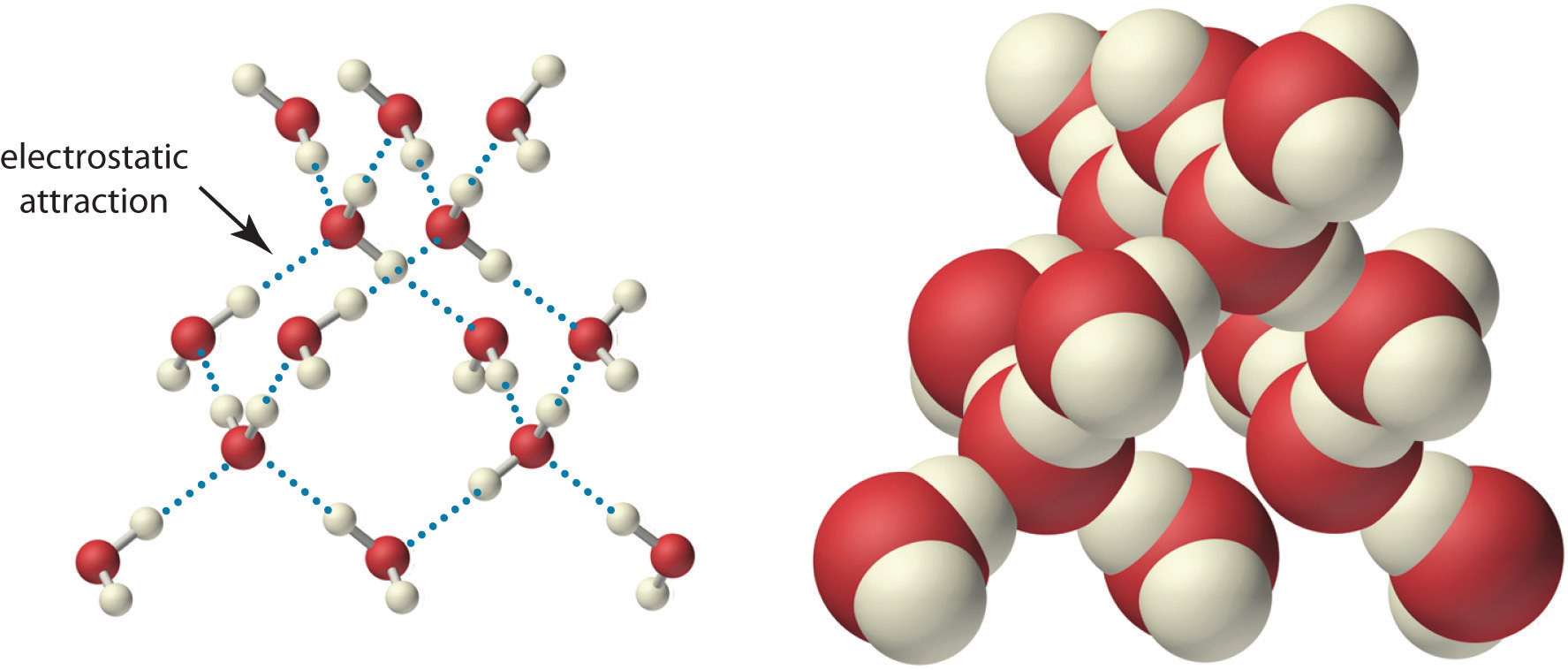
As you learned previously,, ionic compounds such as sodium chloride (NaCl) are also held together by electrostatic interactions—in this case, between oppositely charged ions in the highly ordered solid, where each ion is surrounded by ions of the opposite charge in a fixed arrangement. In contrast to an ionic solid, the structure of liquid water is not completely ordered because the interactions between molecules in a liquid are constantly breaking and reforming.
The unequal charge distribution in polar liquids such as water makes them good solvents for ionic compounds. When an ionic solid dissolves in water, the ions dissociate. That is, the partially negatively charged oxygen atoms of the H2O molecules surround the cations (Na+ in the case of NaCl), and the partially positively charged hydrogen atoms in H2O surround the anions (Cl−; Figure \(\PageIndex{3}\)). Individual cations and anions that are each surrounded by their own shell of water molecules are called hydrated ions. We can describe the dissolution of NaCl in water as
\[\ce{NaCl(s) ->[\ce{H_2O(l)}] Na^{+} (aq) + Cl^{-} (aq)} \label{4.1.1} \]
where (aq) indicates that Na+ and Cl− are hydrated ions.
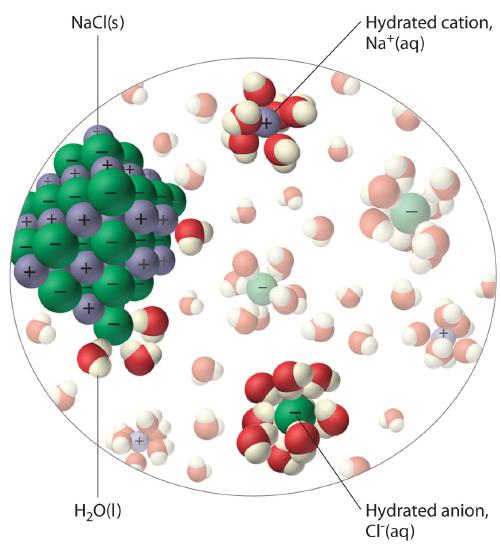
Polar liquids are good solvents for ionic compounds.
Electrolytes
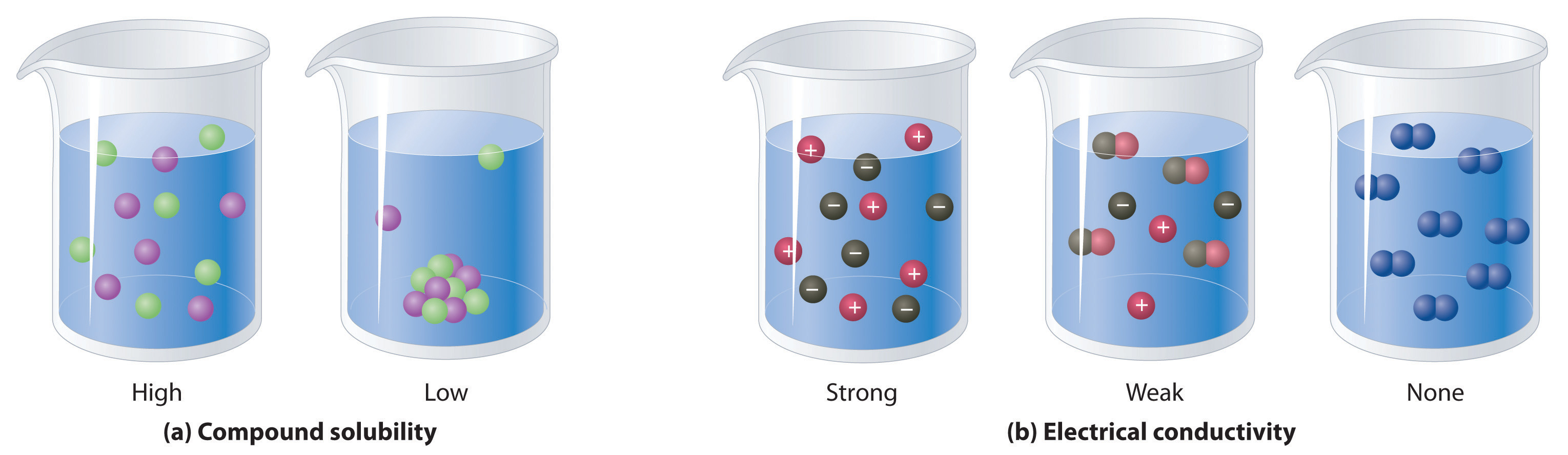
When electricity, in the form of an electrical potential, is applied to a solution, ions in solution migrate toward the oppositely charged rod or plate to complete an electrical circuit, whereas neutral molecules in solution do not (Figure \(\PageIndex{4}\)). Thus solutions that contain ions conduct electricity, while solutions that contain only neutral molecules do not. Electrical current will flow through the circuit shown in Figure \(\PageIndex{4}\) and the bulb will glow only if ions are present. The lower the concentration of ions in solution, the weaker the current and the dimmer the glow. Pure water, for example, contains only very low concentrations of ions, so it is a poor electrical conductor.
Solutions that contain ions conduct electricity.
An electrolyte is any compound that can form ions when dissolved in water. Electrolytes may be strong or weak. When strong electrolytes dissolve, the constituent ions dissociate completely due to strong electrostatic interactions with the solvent, producing aqueous solutions that conduct electricity very well (Figure \(\PageIndex{4}\)). Examples include ionic compounds such as barium chloride (\(\ce{BaCl_2}\)) and sodium hydroxide (\(\ce{NaOH}\)), which are both strong electrolytes and dissociate as follows:
\[\ce{BaCl_2(s) ->[\ce{H_2O(l)}] Ba^{2+} (aq) + 2Cl^{-} (aq)} \label{4.1.2} \]
\[\ce{ NaOH(s) ->[\ce{H_2O(l)}] Na^{+} (aq) + OH^{-} (aq)} \label{4.1.3} \]
The single arrows from reactant to products in Equations \(\ref{4.1.2}\) and \(\ref{4.1.3}\) indicate that dissociation is complete.
When weak electrolytes dissolve, they produce relatively few ions in solution. This does not necessarily mean that the compounds do not dissolve readily in water; many weak electrolytes contain polar bonds and are therefore very soluble in a polar solvent such as water. They do not completely dissociate to form ions, however, because of their weaker electrostatic interactions with the solvent. Because very few of the dissolved particles are ions, aqueous solutions of weak electrolytes do not conduct electricity as well as solutions of strong electrolytes. One such compound is acetic acid (\(\ce{CH3CO2H}\)), which contains the \(\ce{–CO2H}\) unit. Although it is soluble in water, it is a weak acid and therefore also a weak electrolyte. Similarly, ammonia (\(\ce{NH3}\)) is a weak base and therefore a weak electrolyte. The behavior of weak acids and weak bases will be described in more detail later.
Notice that both contain the C=O group.
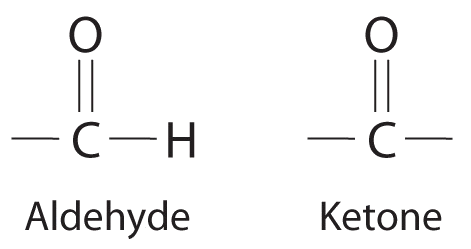
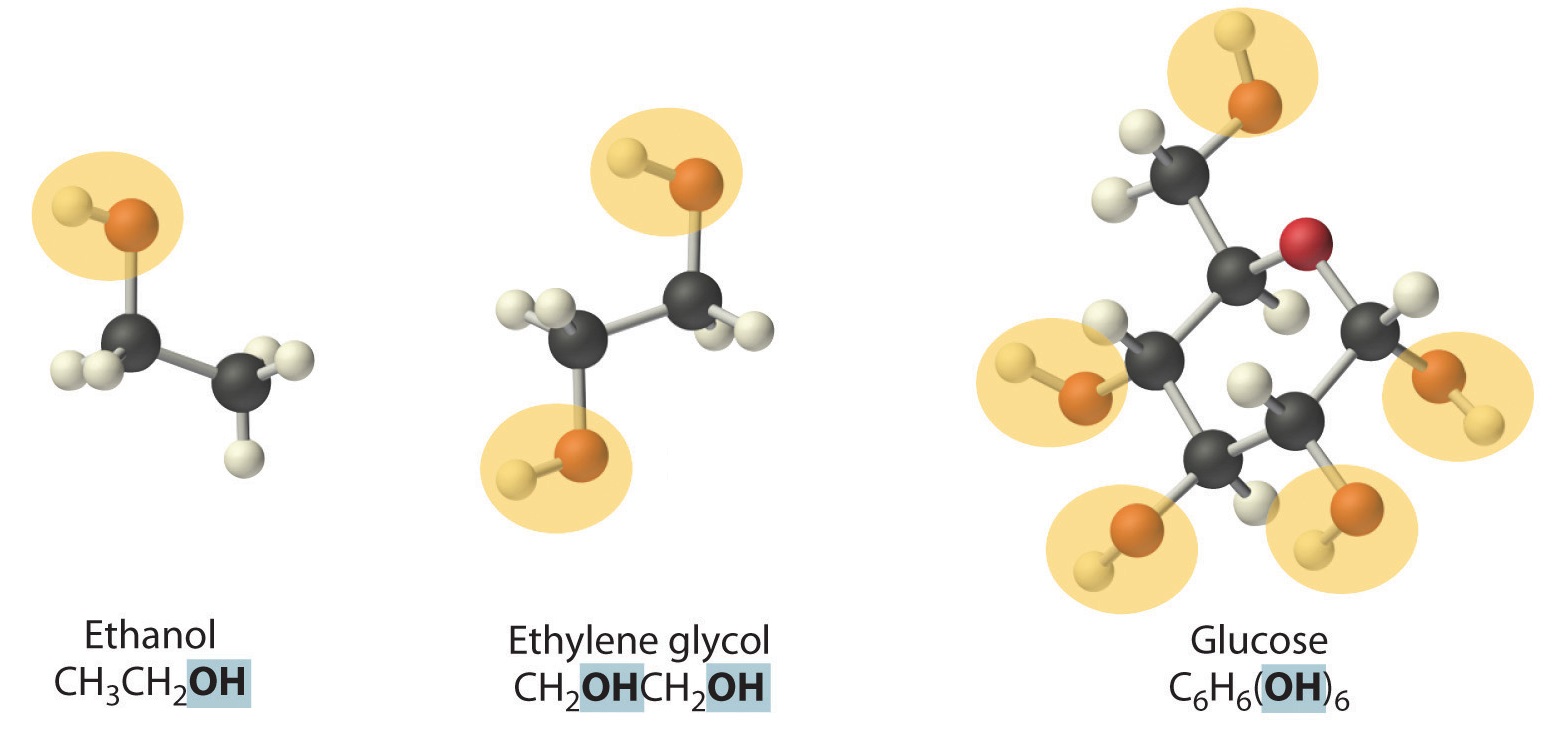
Nonelectrolytes (a substance that dissolves in water to form neutral molecules and has essentially no effect on electrical conductivity) that dissolve in water do so as neutral molecules and thus have essentially no effect on conductivity. Examples of nonelectrolytes that are very soluble in water but that are essentially nonconductive are ethanol, ethylene glycol, glucose, and sucrose, all of which contain the –OH group that is characteristic of alcohols (Figure \(\PageIndex{5}\)). The topic of why alcohols and carboxylic acids behave differently in aqueous solution is for a different Module; for now, however, you can simply look for the presence of the –OH and –CO2H groups when trying to predict whether a substance is a strong electrolyte, a weak electrolyte, or a nonelectrolyte. The distinctions between soluble and insoluble substances and between strong, weak, and nonelectrolytes are illustrated in Figure \(\PageIndex{6}\).
Ionic substances and carboxylic acids are electrolytes; alcohols, aldehydes, and ketones are nonelectrolytes.
Predict whether each compound is a strong electrolyte, a weak electrolyte, or a nonelectrolyte in water.
- formaldehyde
- cesium chloride
Given: compound
Asked for: relative ability to form ions in water
Strategy:
A Classify the compound as ionic or covalent.
B If the compound is ionic and dissolves, it is a strong electrolyte that will dissociate in water completely to produce a solution that conducts electricity well. If the compound is covalent and organic, determine whether it contains the carboxylic acid group. If the compound contains this group, it is a weak electrolyte. If not, it is a nonelectrolyte.
Solution:
- A Formaldehyde is an organic compound, so it is covalent. B It contains an aldehyde group, not a carboxylic acid group, so it should be a nonelectrolyte.
- A Cesium chloride (CsCl) is an ionic compound that consists of Cs+ and Cl− ions. B Like virtually all other ionic compounds that are soluble in water, cesium chloride will dissociate completely into Cs+(aq) and Cl−(aq) ions. Hence it should be a strong electrolyte.
Predict whether each compound is a strong electrolyte, a weak electrolyte, or a nonelectrolyte in water.
- \(\ce{(CH3)2CHOH}\) (2-propanol)
- ammonium sulfate
Answer
- nonelectrolyte
- strong electrolyte
Summary
Aqueous solutions can be classified as polar or nonpolar depending on how well they conduct electricity. Most chemical reactions are carried out in solutions, which are homogeneous mixtures of two or more substances. In a solution, a solute (the substance present in the lesser amount) is dispersed in a solvent (the substance present in the greater amount). Aqueous solutions contain water as the solvent, whereas nonaqueous solutions have solvents other than water. Polar substances, such as water, contain asymmetric arrangements of polar bonds, in which electrons are shared unequally between bonded atoms. Polar substances and ionic compounds tend to be most soluble in water because they interact favorably with its structure. In aqueous solution, dissolved ions become hydrated; that is, a shell of water molecules surrounds them. Substances that dissolve in water can be categorized according to whether the resulting aqueous solutions conduct electricity. Strong electrolytes dissociate completely into ions to produce solutions that conduct electricity well. Weak electrolytes produce a relatively small number of ions, resulting in solutions that conduct electricity poorly. Nonelectrolytes dissolve as uncharged molecules and have no effect on the electrical conductivity of water.


