19.07: Fission
- Page ID
- 178268
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Learning Outcomes
- Define fission.
- Describe a nuclear chain reaction and how it is applied in both a fission bomb and in a nuclear power plant.
- Define fusion.
Nuclear Fission
The most stable nuclei are of intermediate mass. To become more stable, the heaviest nuclei are capable of splitting into smaller fragments. Nuclear fission is a process in which a very heavy nucleus (mass > 200) splits into smaller nuclei of intermediate mass. Because the smaller nuclei are more stable, the fission process releases tremendous amounts of energy. Nuclear fission may occur spontaneously or may occur as a result of bombardment. When uranium-235 is hit with a slow-moving neutron, it absorbs it and temporarily becomes the very unstable uranium-236. This nucleus splits into two medium-mass nuclei while also emitting more neutrons. The mass of the products is less than the mass of the reactants, with the lost mass being converted to energy.
Nuclear Chain Reactions
Because the fission process produces more neutrons, a chain reaction can result. A chain reaction is a reaction in which the material that starts the reaction is also one of the products and can start another reaction. Illustrated below is a nuclear chain reaction for the fission of uranium-235.
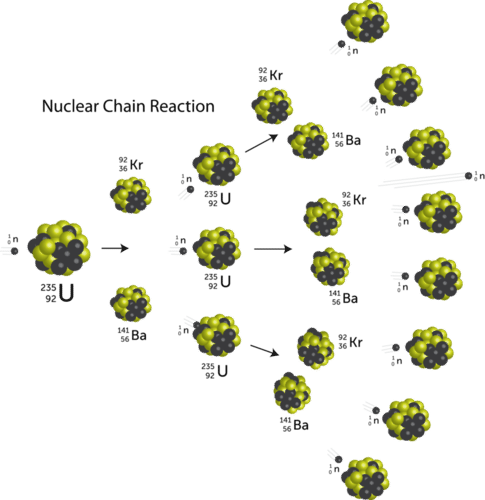
The original uranium-235 nucleus absorbs a neutron, splits into a krypton-92 nucleus and a barium-141 nucleus, and releases three more neutrons upon splitting.
\[\ce{^{235}_{92}U} + \ce{^1_0n} \rightarrow \ce{^{92}_{36}Kr} + \ce{^{141}_{56}Ba} + 3 \ce{^1_0n}\]
Those three neutrons are then able to cause the fission of three more uranium-235 nuclei, each of which release more neutrons, and so on. The chain reaction continues until all of the uranium-235 nuclei have been split, or until the released neutrons escape the sample without striking any more nuclei. If the size of the original sample of uranium-235 is sufficiently small, too many neutrons escape without striking other nuclei, and the chain reaction quickly ceases. The critical mass is the minimum amount of fissionable material needed to sustain a chain reaction. Atomic bombs and nuclear reactors are two ways to harness the large energy released during nuclear fission.
Atomic Bombs - Uncontrolled Nuclear Reactions
In an atomic bomb, or fission bomb, the nuclear chain reaction is designed to be uncontrolled, releasing huge amounts of energy in a short amount of time. A critical mass of fissionable plutonium is contained within the bomb, but not at a sufficient density. Conventional explosives are used to compress the plutonium, causing it to go critical and trigger a nuclear explosion.
Nuclear Power Plants- Controlled Nuclear Reactions
A nuclear power plant (see figure below) uses a controlled fission reaction to produce large amounts of heat. The heat is then used to generate electrical energy.
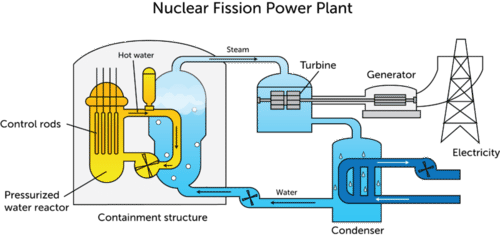
Uranium-235, the usual fissionable material in a nuclear reactor, is first packaged into fuel rods. In order to keep the chain reaction from processing unchecked, moveable control rods are placed in between the fuel rods. Control rods limit the amount of available neutrons by absorbing some of them and preventing the reaction from proceeding too rapidly. Common control rod materials include alloys with various amounts of silver, indium, cadmium, or boron. A moderator is a material that slows down high-speed neutrons. This is beneficial because slow-moving neutrons are more efficient at splitting nuclei. Water is often used as a moderator. The heat released by the fission reaction is absorbed by constantly circulating coolant water. The coolant water releases its heat to a steam generator, which turns a turbine and generates electricity. The core of the reactor is surrounded by a containment structure that absorbs radiation.
Contributors
Allison Soult, Ph.D. (Department of Chemistry, University of Kentucky)


