Chapter 1.5: The Atom
- Page ID
- 17280
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)
Learning Objectives
- To become familiar with the components and structure of the atom.
To date, about 115 different elements have been discovered; by definition, each is chemically unique. To understand why they are unique, you need to understand the structure of the atom (the fundamental, individual particle of an element) and the characteristics of its components.
Atoms consist of electrons, a subatomic particle with a negative charge that resides around the nucleus of all atoms. protons, a subatomic particle with a positive charge that resides in the nucleus of all atoms. and neutrons, a subatomic particle with no charge that resides in the nucleus of almost all atoms..This is an oversimplification that ignores the other subatomic particles that have been discovered, but it is sufficient for our discussion of chemical principles. Some properties of these subatomic particles are summarized in Table 1.5.1 , which illustrates three important points.
- Electrons and protons have electrical charges that are identical in magnitude but opposite in sign. No particle with any fraction charge has ever been discovered although many have tried. For historical reasons having to do with the earliest studies of electricity, we assign charges of −1 and +1 to the electron and proton, respectively.
- Neutrons have approximately the same mass as protons but no charge. They are electrically neutral.
- The mass of a proton or a neutron is about 1836 times greater than the mass of an electron. Protons and neutrons constitute by far the bulk of the mass of atoms.
The discovery of the electron and the proton was crucial to the development of the modern model of the atom and provides an excellent case study in the application of the scientific method. In fact, the elucidation of the atom’s structure is one of the greatest detective stories in the history of science.
Table 1.5.1 Properties of Subatomic Particles*
| Particle | Mass (g) | Atomic Mass (amu) | Electrical Charge (coulombs) | Relative Charge |
|---|---|---|---|---|
| electron | 9.109 × 10−28 | 0.0005486 | −1.602 × 10−19 | −1 |
| proton | 1.673 × 10−24 | 1.007276 | +1.602 × 10−19 | +1 |
| neutron | 1.675 × 10−24 | 1.008665 | 0 | 0 |
| * For a review of using scientific notation and units of measurement, see Essential Skills 1 (Section 1.8 "Essential Skills 1). | ||||
The Electron
Figure 1.5.1 A Gas Discharge Tube Producing Cathode Rays When a high voltage is applied to a gas contained at low pressure in a gas discharge tube, electricity flows through the gas, and energy is emitted in the form of light. Neon signs work this way
Long before the end of the 19th century, it was well known that applying a high voltage to a gas contained at low pressure in a sealed tube (called a gas discharge tube) caused electricity to flow through the gas, which then emitted light (Figure 1.5.1 ). Researchers trying to understand this phenomenon found that an unusual form of energy was also emitted from the cathode, or negatively charged electrode; hence this form of energy was called cathode rays. In 1897, the British physicist J. J. Thomson (1856–1940) proved that atoms were not the ultimate form of matter. He demonstrated that cathode rays could be deflected, or bent, by magnetic or electric fields, which indicated that cathode rays consist of charged particles (Figure 1.5.2 ). More important, by measuring the extent of the deflection of the cathode rays in magnetic or electric fields of various strengths, Thomson was able to calculate the mass-to-charge ratio of the particles. These particles were emitted by the negatively charged cathode and repelled by the negative terminal of an electric field. Because like charges repel each other and opposite charges attract, Thomson concluded that the particles had a net negative charge; we now call these particles electrons. Most important for chemistry, Thomson found that the mass-to-charge ratio of cathode rays was independent of the nature of the metal electrodes or the gas, which suggested that electrons were fundamental components of all atoms.
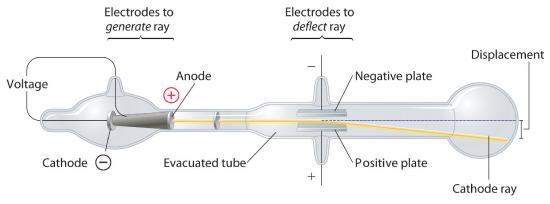
Figure 1.5. 2 Deflection of Cathode Rays by an Electric Field As the cathode rays travel toward the right, they are deflected toward the positive electrode (+), demonstrating that they are negatively charged.
The video below shows how JJ Thompson used such a tube to measure the ration of charge over mass of an electron
Figure 1.5.3 Measuring e/m For an ElectronVideo from Davidson College demonstrating Thompson's e/m experiment. You can also use an applet from the Google Group Physics Flash to simulate the experiment and here is another applet, from Kings Center for Visual Exploration in Alberta (KCVE) with a detailed explanation
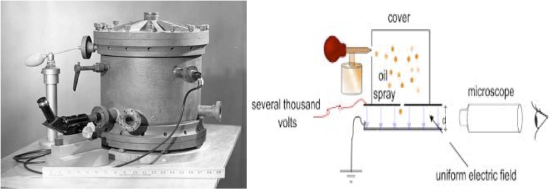
Subsequently, the American scientist Robert Millikan (1868–1953) carried out a series of experiments using electrically charged oil droplets, which allowed him to calculate the charge on a single electron. Millikan sprayed oil drops into a jar past a radioactive source which ionized some of them. By balancing the gravitational force on the ionized drops causing them to fall, with an electric force generated by placing a voltage difference between the top and bottom of the jar, Millikan was able to calculate the charge on the drops. He found that there was a minimum charge, and that all of the drops either had the same charge or an integral number of such. This is the charge on the electron.
Figure 1.5.4 Millikan Oil Drop ExperimentMiliikan's apparatus is shown on the left, a schematic drawing of it on the right. See if you can identify the different parts. You can find a Millikan applet on KVCS or you another applet at Davidson College
And here is a video explanation from Russell Lidgard
With this information and Thomson’s mass-to-charge ratio, Millikan determined the mass of an electron:
\[\dfrac {mass}{charge} \times {charge} ={mass}\]
where the charge cancels. It was at this point that two separate lines of investigation began to converge, both aimed at determining how and why matter emits energy.
Radioactivity
The second line of investigation began in 1896, when the French physicist Henri Becquerel (1852–1908) discovered that certain minerals, such as uranium salts, emitted a new form of energy. Becquerel’s work was greatly extended by Marie Curie (1867–1934) and her husband, Pierre (1854–1906); all three shared the Nobel Prize in Physics in 1903. Marie Curie coined the term radioactivity (from the Latin radius, meaning “ray”) to describe the emission of energy rays by matter. She found that one particular uranium ore, pitchblende, was substantially more radioactive than most, which suggested that it contained one or more highly radioactive impurities. Starting with several tons of pitchblende, the Curies isolated two new radioactive elements after months of work: polonium, which was named for Marie’s native Poland, and radium, which was named for its intense radioactivity. Pierre Curie carried a vial of radium in his coat pocket to demonstrate its greenish glow, a habit that caused him to become ill from radiation poisoning well before he was run over by a horse-drawn wagon and killed instantly in 1906. Marie Curie, in turn, died of what was almost certainly radiation poisoning.
.
Figure 1.5.6 Radium bromide illuminated by its own radioactive glow This 1922 photo was taken in the dark in the Curie laboratory.
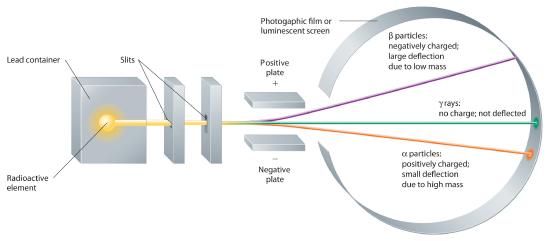
Building on the Curies’ work, the British physicist Ernest Rutherford (1871–1937) performed decisive experiments that led to the modern view of the structure of the atom. While working in Thomson’s laboratory shortly after Thomson discovered the electron, Rutherford showed that compounds of uranium and other elements emitted at least two distinct types of radiation. One was readily absorbed by matter and seemed to consist of particles that had a positive charge and were massive compared to electrons. Because it was the first kind of radiation to be discovered, Rutherford called these substances α particles. Rutherford also showed that the particles in the second type of radiation, β particles, had the same charge and mass-to-charge ratio as Thomson’s electrons; they are now known to be high-speed electrons. A third type of radiation, γ rays, was discovered somewhat later and found to be similar to a form of radiation called x-rays, now used to produce images of bones and teeth.
These three kinds of radiation—α particles, β particles, and γ rays—are readily distinguished by the way they are deflected by an electric field and by the degree to which they penetrate matter. As Figure 1.5.7 illustrates, α particles and β particles are deflected in opposite directions; α particles are deflected to a much lesser extent because of their higher mass-to-charge ratio. In contrast, γ rays have no charge, so they are not deflected by electric or magnetic fields. Figure 1.5.8 shows that α particles have the least penetrating power and are stopped by a sheet of paper, whereas β particles can pass through thin sheets of metal but are absorbed by lead foil or even thick glass. In contrast, γ-rays can readily penetrate matter; thick blocks of lead or concrete are needed to stop them.
Figure 1.5.7 Effect of an Electric Field on α Particles, β Particles, and γ RaysA negative electrode deflects negatively charged β particles, whereas a positive electrode deflects positively charged α particles. Uncharged γ rays are unaffected by an electric field. (Relative deflections are not shown to scale.)
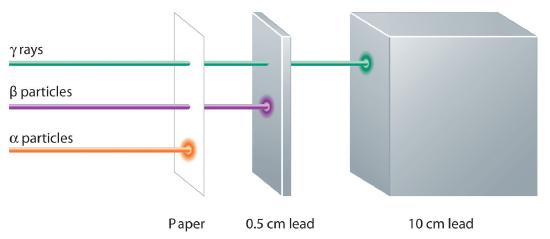
Figure 1.5.8 Relative Penetrating Power of the Three Types of RadiationA sheet of paper stops comparatively massive α particles, whereas β particles easily penetrate paper but are stopped by a thin piece of lead foil. Uncharged γ rays penetrate the paper and lead foil; a much thicker piece of lead or concrete is needed to absorb them.
The Atomic Model
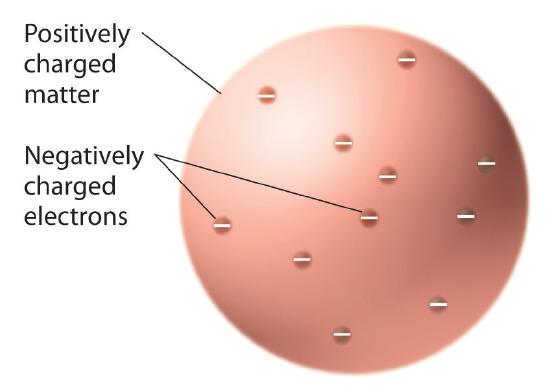
Once scientists concluded that all matter contains negatively charged electrons, it became clear that atoms, which are electrically neutral, must also contain positive charges to balance the negative ones. Thomson proposed that the electrons were embedded in a uniform sphere that contained both the positive charge and most of the mass of the atom, much like raisins in plum pudding or chocolate chips in a cookie (Figure 1.5.9 ).
Figure 1.5.9 Thomson’s Plum Pudding or Chocolate Chip Cookie Model of the AtomIn this model, the electrons are embedded in a uniform sphere of positive charge.
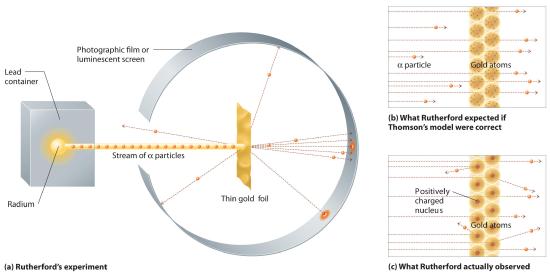
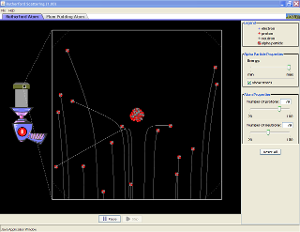
In a single famous experiment, however, Rutherford showed unambiguously that Thomson’s model of the atom was impossible. Rutherford aimed a stream of α particles at a very thin gold foil target (part (a) in Figure 1.5.10 ) and examined how the α particles were scattered by the foil. Gold was chosen because it could be easily hammered into extremely thin sheets with a thickness that minimized the number of atoms in the target. If Thomson’s model of the atom were correct, the positively charged α particles should crash through the uniformly distributed mass of the gold target like cannonballs through the side of a wooden house. They might be moving a little slower when they emerged, but they should pass essentially straight through the target (part (b) in Figure 1.5.10 ). To Rutherford’s amazement, a small fraction of the α particles were deflected at large angles, and some were reflected directly back at the source (part (c) in Figure 1.5.10). According to Rutherford, “It was almost as incredible as if you fired a 15-inch shell at a piece of tissue paper and it came back and hit you.”
Figure 1.5.10 A Summary of Rutherford’s Experiments(a) A representation of the apparatus Rutherford used to detect deflections in a stream of α particles aimed at a thin gold foil target. The particles were produced by a sample of radium. (b) If Thomson’s model of the atom were correct, the α particles should have passed straight through the gold foil. (c) But a small number of α particles were deflected in various directions, including right back at the source. This could be true only if the positive charge were much more massive than the α particle. It suggested that the mass of the gold atom is concentrated in a very small region of space, which he called the nucleus.
You can run a simulation of the Rutherford Scattering Experiment
Rutherford’s results were not consistent with a model in which the mass and positive charge are distributed uniformly throughout the volume of an atom. Instead, they strongly suggested that both the mass and positive charge are concentrated in a tiny fraction of the volume of an atom, which Rutherford called the nucleusThe central core of an atom where protons and any neutrons reside.. It made sense that a small fraction of the α particles collided with the dense, positively charged nuclei in either a glancing fashion, resulting in large deflections, or almost head-on, causing them to be reflected straight back at the source.
KCVS has more detail about these experiments as well as a series of applets that you can use
Although Rutherford could not explain why repulsion between the positive charges in nuclei that contained more than one positive charge did not cause the nucleus to disintegrate, he reasoned that repulsion between negatively charged electrons would cause the electrons to be uniformly distributed throughout the atom’s volume.Today we know that strong nuclear forces, which are much stronger than electrostatic interactions, hold the protons and the neutrons together in the nucleus. For this and other insights, Rutherford was awarded the Nobel Prize in Chemistry in 1908. Unfortunately, Rutherford would have preferred to receive the Nobel Prize in Physics because he thought that physics was superior to chemistry. In his opinion, “All science is either physics or stamp collecting.” (The authors of this text do not share Rutherford’s view!)
Subsequently, Rutherford established that the nucleus of the hydrogen atom was a positively charged particle, for which he coined the name proton in 1920. He also suggested that the nuclei of elements other than hydrogen must contain electrically neutral particles with approximately the same mass as the proton. The neutron, however, was not discovered until 1932, when James Chadwick (1891–1974, a student of Rutherford; Nobel Prize in Physics, 1935) discovered it. As a result of Rutherford’s work, it became clear that an α particle contains two protons and neutrons and is therefore simply the nucleus of a helium atom.
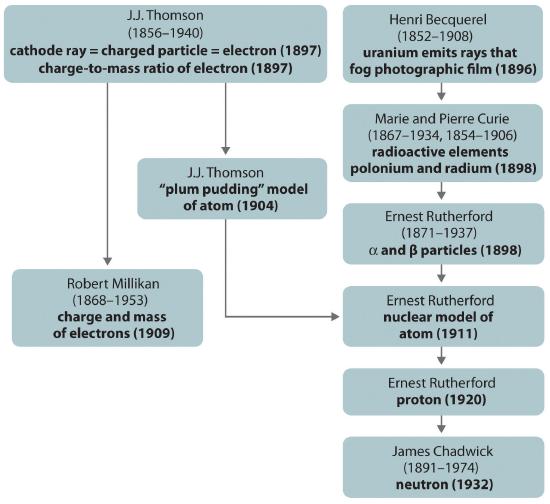
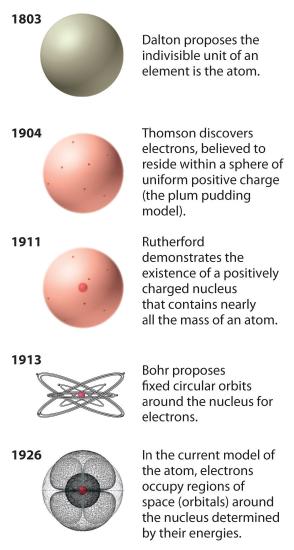
The historical development of the different models of the atom’s structure is summarized in Figure 1.5.11 Rutherford’s model of the atom is essentially the same as the modern one, except that we now know that electrons are not uniformly distributed throughout an atom’s volume. Instead, they are distributed according to a set of principles described in Chapter 2 "The Structure of Atoms". Figure 1.5.12 shows how the model of the atom has evolved over time from the indivisible unit of Dalton to the modern view taught today.
Figure 1.5.11 A Summary of the Historical Development of Models of the Components and Structure of the Atom The dates in parentheses are the years in which the key experiments were performed.
Figure 1.5.12 The Evolution of Atomic Theory, as Illustrated by Models of the Oxygen AtomBohr’s model and the current model are described in Chapter 2 "The Structure of Atoms".
Summary
Atoms, the smallest particles of an element that exhibit the properties of that element, consist of negatively charged electrons around a central nucleus composed of more massive positively charged protons and electrically neutral neutrons. Radioactivity is the emission of energetic particles and rays (radiation) by some substances. Three important kinds of radiation are α particles (helium nuclei), β particles (electrons traveling at high speed), and γ rays (similar to x-rays but higher in energy).
Key Takeaway
- The atom consists of discrete particles that govern its chemical and physical behavior.
Conceptual Problems
-
Describe the experiment that provided evidence that the proton is positively charged.
-
What observation led Rutherford to propose the existence of the neutron?
-
What is the difference between Rutherford’s model of the atom and the model chemists use today?
-
If cathode rays are not deflected when they pass through a region of space, what does this imply about the presence or absence of a magnetic field perpendicular to the path of the rays in that region?
-
Describe the outcome that would be expected from Rutherford’s experiment if the charge on α particles had remained the same but the nucleus were negatively charged. If the nucleus were neutral, what would have been the outcome?
-
Describe the differences between an α particle, a β particle, and a γ ray. Which has the greatest ability to penetrate matter?
Numerical Problems
Please be sure you are familiar with the topics discussed in Essential Skills 1 (Section 1.8 "Essential Skills 1) before proceeding to the Numerical Problems.
-
Using the data in Table 1.5.1 and the periodic table, calculate the percentage of the mass of a helium atom that is due to
- electrons.
- protons.
-
The radius of an atom is approximately 104 times larger than the radius of its nucleus. If the radius of the nucleus were 1.0 cm, what would be the radius of the atom in centimeters? in miles?
-
The total charge on an oil drop was found to be 3.84 × 10−18 coulombs. What is the total number of electrons contained in the drop?
Contributors
- Anonymous
Modified by Joshua Halpern (Howard University)
e/m Experiment Video from Carleton College on YouTube
Millikan Oil Drop Experiment Video from Robert Lidgard on YouTube
Rutherford Scattering Experiment Applet from pHet