Chapter 1.3: A Description of Matter
- Page ID
- 17278
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)
Learning Objectives
- To classify matter.
Chemists study the structures, physical properties, and chemical properties of material substances. These consist of matter which is anything that occupies space and has mass. Gold and iridium are matter, as are peanuts, people, and postage stamps. Smoke, smog, and laughing gas are matter. Energy, light, and sound, however, are not matter; ideas and emotions are also not matter.
We all know about E=mc2, how Einstein discovered that mass and energy are interchangeable. However the amount of energy released in conversion of even the tiniest amount of mass to energy is far beyond normal chemistry. For practical purposes atoms (mass) are not transmuted either into other types of atoms or to pure energy. We can and do do this in nuclear reactors, atom smashers and atomic weapons, however, these are not normal, everyday chemistry, and the energy range involved in nuclear chemistry and such processes is well beyond common experience and this course.
We know about four forces in the universe. Gravity is a very weak force that functions on the scale of galaxys as well as apples falling. Gravitational effects are only obvious for very large objects, such as the Earth, the Sun, galaxies and the universe. There is very little in chemistry where we have to include gravity in our analysis, well other than in how we weigh chemicals.
Most chemical phenomina are controlled by electromagnetic forces, the attraction and repulsion of small sub-atomic particles such as electrons and nucleii for each other. The typical distances involved in the interactions of these charged particles are a billionth of a meter.
The weak and strong forces are forces holding nuclei together. These forces are studied by nuclear and high energy physicists. Their range is much smaller than the electromagnetic force, in the case of the weak interaction 10-17 m, roughly the size of the atomic nucleus.
The strong force, is also short range, but also very strong. It is what holds atomic nuclei together and confines quarks in nuclear particles such as protons and neutrons. Again, like gravitation, there is very little in chemistry where we need to consider the strong and weak forces in our analysis.
The mass of an object is the quantity of matter it contains. Do not confuse an object’s mass with its weight. The weight of an object depends on its location (c.f. mass), which is a force caused by the gravitational attraction between different bodies. Mass is a fundamental property of an object that does not depend on its location. In physical terms, the mass of an object is directly proportional to the force required to change its speed or direction. Weight, on the other hand, depends on the location of an object relative to other bodies, such as the Earth. An astronaut whose mass is 95 kg weighs about 210 lb on Earth but only about 35 lb on the moon because the gravitational force he or she experiences on the moon is approximately one-sixth the force experienced on Earth. This is because the Earth has much more mass than the moon. For practical purposes, weight and mass are often used interchangeably in laboratories, that is one talks about mass in kg, in the same manner as weight in kg but fundamentally kilograms are units of mass, not weight. Nominally, the force of gravity is considered to be the same everywhere on Earth’s surface so the weight of a 2.0 kg mass will be twice the weight of a 1.0 kg mass.
Under normal conditions, there are three distinct states of matter: solids, liquids, and gases (Figure 1.3.1 ). Solids are relatively rigid and have fixed shapes and volumes. A rock, for example, is a solid. In contrast, liquids have fixed volumes but flow to assume the shape of their containers, such as a beverage in a can. Gases, such as air, have neither fixed shapes nor fixed volumes and expand to completely fill their containers. Whereas the volume of gases strongly depends on their temperature and pressure (the amount of force exerted on a given area), the volumes of liquids and solids are virtually independent of temperature and pressure. Matter can often change from one physical state to another in a process called a physical change. For example, liquid water can be heated to form a gas called steam, or steam can be cooled to form liquid water. However, such changes of state do not affect the chemical composition of the substance.
Figure 1.3.1 The Three States of Matter Solids have a defined shape and volume. Liquids have a fixed volume but flow to assume the shape of their containers. Gases completely fill their containers, regardless of volume. A bar of gold is shown to the left, liquid gold being poured in the center, and an ampule of liquid bromine with gaseous bromine at the right
Pure Substances and Mixtures
A pure chemical substance is any matter that has a fixed chemical composition and characteristic properties. Oxygen, for example, is a pure chemical substance that is a colorless, odorless gas at 25°C. Very few samples of matter consist of pure substances; instead, most are mixtures which are combinations of two or more pure substances in variable proportions in which the individual substances retain their identity. Air, tap water, milk, blue cheese, bread, and dirt are all mixtures. If all portions of a material are in the same state, have no visible boundaries, and are uniform throughout, then the material is homogeneous. Examples of homogeneous mixtures are the air we breathe and the tap water we drink. Homogeneous mixtures are also called solutions. Thus air is a solution of nitrogen, oxygen, water vapor, carbon dioxide, and several other gases; tap water is a solution of small amounts of several substances in water. The specific compositions of both of these solutions are not fixed, however, but depend on both source and location; for example, the composition of tap water in Boise, Idaho, is not the same as the composition of tap water in Buffalo, New York. Although most solutions we encounter are liquid, solutions can also be solid. The gray substance still used by some dentists to fill tooth cavities is a complex solid solution that contains 50% mercury and 50% of a powder that contains silver, tin, and copper, with small amounts of zinc and mercury. Solid solutions of two or more metals are commonly called alloys.
If the composition of a material is not completely uniform, then it is heterogeneous (e.g., chocolate chip cookie dough, blue cheese, and dirt). Mixtures that appear to be homogeneous are often found to be heterogeneous after microscopic examination. Milk, for example, appears to be homogeneous, but when examined under a microscope, it clearly consists of tiny globules of fat and protein dispersed in water. (Figure 1.3.2 ). The components of heterogeneous mixtures can usually be separated by simple physical means. Solid-liquid mixtures such as sand in water or tea leaves in tea are readily separated by filtration, which consists of passing the mixture through a barrier, such as a strainer, with holes or pores that are smaller than the solid particles. In principle, mixtures of two or more solids, such as sugar and salt, can be separated by microscopic inspection and sorting. More complex operations are usually necessary, though, such as when separating gold nuggets from river gravel by panning. First solid material is filtered from river water; then the solids are separated by inspection. If gold is embedded in rock, it may have to be isolated using chemical methods
Homogeneous mixtures (solutions) can be separated into their component substances by physical processes that rely on differences in some physical property, such as differences in their boiling points. Two of these separation methods are distillation and crystallization. Distillation is a physical process used to separate homogeneous mixtures (solutions) into their component substances. Distillation makes use of differences in the volatilities of the component substances. makes use of differences in volatility, a measure of how easily a substance is converted to a gas at a given temperature. Figure 1.3.3 shows a simple distillation apparatus for separating a mixture of substances, at least one of which is a liquid. The most volatile component boils first and is condensed back to a liquid in the water-cooled condenser, from which it flows into the receiving flask. If a solution of salt and water is distilled, for example, the more volatile component, pure water, collects in the receiving flask, while the salt remains in the distillation flask.
Figure 1.3.3 The Distillation of a Solution The solution of salt in water is heated in the distilling flask until it boils. The resulting vapor is enriched in the volatile component (water), which condenses to a liquid in the cold condenser and is then collected in the receiving flask.
Mixtures of two or more liquids with different boiling points can be separated with a more complex distillation apparatus. One example is the refining of crude petroleum into a range of useful products: aviation fuel, gasoline, kerosene, diesel fuel, and lubricating oil (in the approximate order of decreasing volatility). Another example is the distillation of alcoholic spirits such as brandy or whiskey. This relatively simple procedure caused more than a few headaches for federal authorities in the 1920s during the era of Prohibition, when illegal stills proliferated in remote regions of the United States.
Distillation is a basic tool of chemists. Below is a video showing how a distillation apparatus can be set up in Organic Chemistry Lab, something that many of you will need to know next year
Distillation is used in many chemical applications. The art of distillation goes back thousands of years, and among other things is used to extract perfumes from plants such as lavender.
Crystallization is another physical process used to separate homogeneous mixtures (solutions) into their component substances. Crystallization separates mixtures based on differences in their solubilities. separates mixtures based on differences in solubility, a measure of how much solid substance remains dissolved in a given amount of a specified liquid. Most substances are more soluble at higher temperatures, so a mixture of two or more substances can be dissolved at an elevated temperature and then allowed to cool slowly. Alternatively, the liquid, called the solvent, may be allowed to evaporate. In either case, the least soluble of the dissolved substances, the one that is least likely to remain in solution, usually forms crystals first, and these crystals can be removed from the remaining solution by filtration. Figure 1.3.4 dramatically illustrates the process of crystallization.
Figure 1.3.4 The Crystallization of Sodium Acetate from a Concentrated Solution of Sodium Acetate in Water.The addition of a small “seed” crystal causes the compound to form white crystals, which grow and eventually occupy most of the flask.
Most mixtures can be separated into pure substances, which may be either elements or compounds. An element is a pure substance that cannot be broken down into a simpler substance by chemical changes. An example would be metallic sodium. A compound is a pure substance that contains two or more elements and has chemical and physical properties that are usually different from those of the elements of which it is composed. An example of a compound would be crystalline sodium chloride, which contains sodium and chlorine and has chemical and physical properties that are different from both of the elements of which it is composed. With only a few exceptions, a particular compound has the same elemental composition (the same elements in the same proportions) regardless of its source or history. The chemical composition of a substance is altered in a process called a chemical change. The conversion of two or more elements, such as sodium and chlorine, to a chemical compound, sodium chloride, is an example of a chemical change, often called a chemical reaction. Currently, about 115 elements are known, but millions of chemical compounds have been prepared from these 115 elements. The known elements are listed in the periodic table..
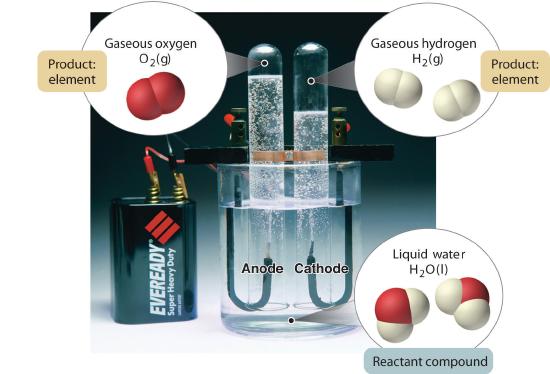
In general, a reverse chemical process breaks down compounds into their elements. For example, water (a compound) can be decomposed into hydrogen and oxygen (both elements) by a process called electrolysis. In electrolysis, electricity provides the energy needed to separate a compound into its constituent elements (Figure 1.3.5 ). A similar technique is used on a vast scale to obtain pure aluminum, an element, from its ores, which are mixtures of compounds. Because a great deal of energy is required for electrolysis, the cost of electricity is by far the greatest expense incurred in manufacturing pure aluminum. Thus recycling aluminum is both cost-effective and ecologically sound.
Figure 1.3.5 The Decomposition of Water to Hydrogen and Oxygen by Electrolysis Water is a chemical compound; hydrogen and oxygen are elements.
This is actually an experiment that (with some care and safety googles) could be done at home.
Think about how we could identify the oxygen and hydrogen?
The overall organization of matter and the methods used to separate mixtures are summarized in Figure 1.3.6
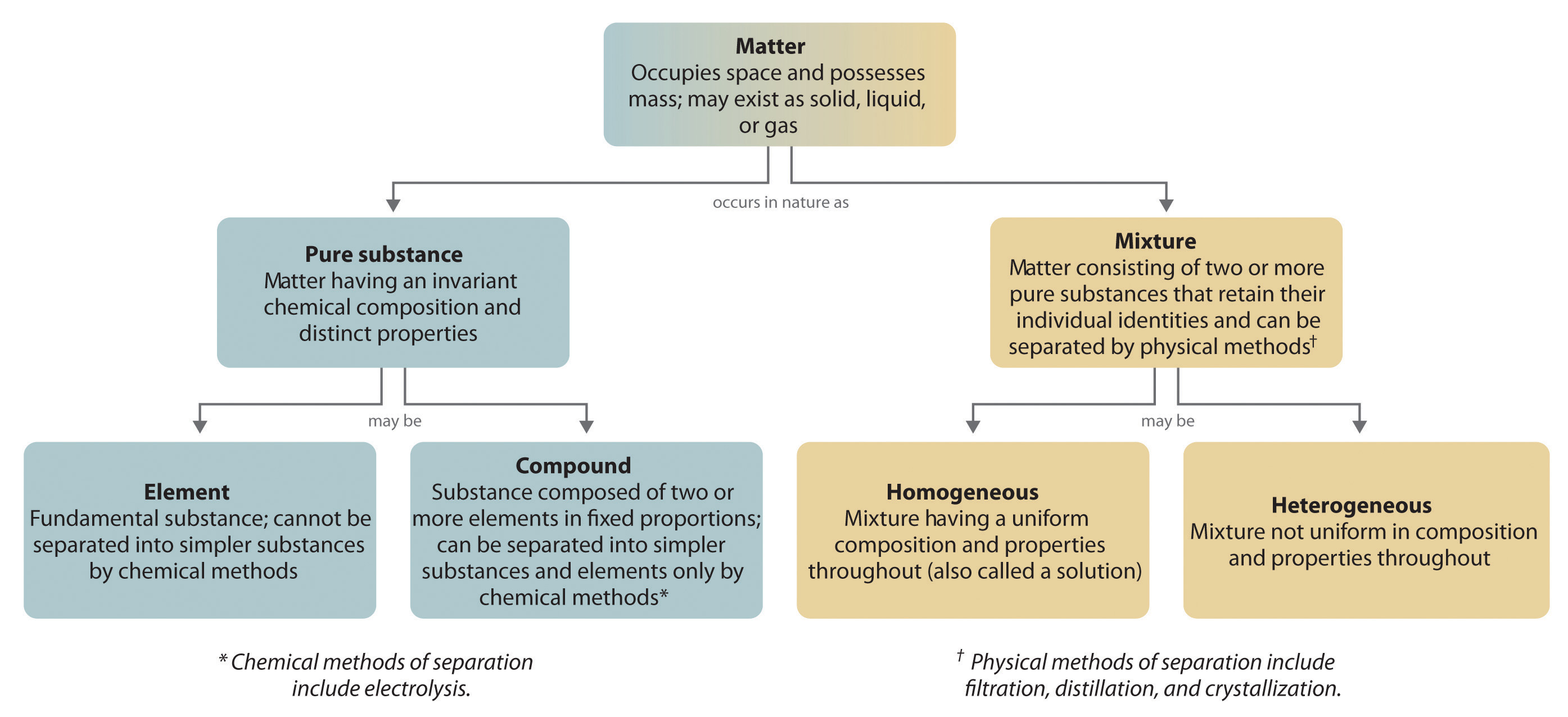
Figure 1.3.6 Relationships between the types of matter and the methods used to separate mixtures
Example 1.3.1
Identify each substance as a compound, an element, a heterogeneous mixture, or a homogeneous mixture (solution).
- filtered tea
- freshly squeezed orange juice
- a compact disc
- aluminum oxide, a white powder that contains a 2:3 ratio of aluminum and oxygen atoms
- selenium
Given: a chemical substance
Asked for: its classification
Strategy:
A Decide whether a substance is chemically pure. If it is pure, the substance is either an element or a compound. If a substance can be separated into its elements, it is a compound.
B If a substance is not chemically pure, it is either a heterogeneous mixture or a homogeneous mixture. If its composition is uniform throughout, it is a homogeneous mixture.
Solution:
- A Tea is a solution of compounds in water, so it is not chemically pure. It is usually separated from tea leaves by filtration. B Because the composition of the solution is uniform throughout, it is a homogeneous mixture.
- A Orange juice contains particles of solid (pulp) as well as liquid; it is not chemically pure. B Because its composition is not uniform throughout, orange juice is a heterogeneous mixture.
- A A compact disc is a solid material that contains more than one element, with regions of different compositions visible along its edge. Hence a compact disc is not chemically pure. B The regions of different composition indicate that a compact disc is a heterogeneous mixture.
- A Aluminum oxide is a single, chemically pure compound.
- A Selenium is one of the known elements.
Exercise
Identify each substance as a compound, an element, a heterogeneous mixture, or a homogeneous mixture (solution).
- white wine
- mercury
- ranch-style salad dressing
- table sugar (sucrose)
Answer:
- solution
- element
- heterogeneous mixture
- compound
Properties of Matter
All matter has physical and chemical properties. Physical properties A characteristic that scientists can measure without changing the composition of a sample under study. are characteristics that scientists can measure without changing the composition of the sample under study, such as mass, color, and volume (the amount of space occupied by a sample). Chemical properties The characteristic ability of a substance to react to form new substances. describe the characteristic ability of a substance to react to form new substances; they include its flammability and susceptibility to corrosion. All samples of a pure substance have the same chemical and physical properties. For example, pure copper is always a reddish-brown solid (a physical property) and always dissolves in dilute nitric acid to produce a blue solution and a brown gas (a chemical property).
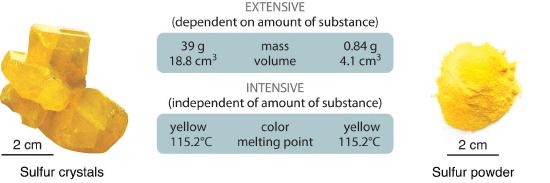
Physical properties can be extensive or intensive. Extensive properties A physical property that varies with the amount of a substance. vary with the amount of the substance and include mass, weight, and volume. Intensive properties A physical property that does not depend on the amount of the substance and physical state at a given temperature and pressure., in contrast, do not depend on the amount of the substance; they include color, melting point, boiling point, electrical conductivity, and physical state at a given temperature. For example, elemental sulfur is a yellow crystalline solid that does not conduct electricity and has a melting point of 115.2°C, no matter what amount is examined (Figure 1.3.7 ). Scientists commonly measure intensive properties to determine a substance’s identity, whereas extensive properties convey information about the amount of the substance in a sample.
Figure 1.3.7 The Difference between Extensive and Intensive Properties of Matter. Because they differ in size, the two samples of sulfur have different extensive properties, such as mass and volume. In contrast, their intensive properties, including color, melting point, and electrical conductivity, are identical.
Although mass and volume are both extensive properties, their ratio is an important intensive property called density (d) . Density is defined as mass per unit volume and is usually expressed in grams per cubic centimeter (g/cm3). At a given temperature, the density of a substance is a constant. As mass increases in a given volume, density also increases. For example, lead, with its greater mass, has a far greater density than the same volume of air, just as a brick has a greater density than the same volume of Styrofoam. At a given temperature and pressure, the density of a pure substance is a constant:
\( density= mass/volume\rightarrow d=m/V \tag{1.3.1} \)
Pure water, for example, has a density of 0.998 g/cm3 at 25°C. You can get some feel about density using the density applet from PheT
If you want some practice with density problems, here are some web resources
These are videos showing how to solve density problems
Math Tutor explains density, and does some problems
Video Chemistry Textbook Example 1, Example 2, Example 3
and here are some problem sets for practice
From Scott van Bramer Widener University
From Carleton University
From the Algebra Lab
The average densities of some common substances are in Table 1.3.1 . Notice that corn oil has a lower mass to volume ratio than water. This means that when added to water, corn oil will “float.” Example 3 shows how density measurements can be used to identify pure substances.
Table 1.3.1 Densities of Common Substances
| Substance | Density at 25°C (g/cm3) |
|---|---|
| blood | 1.035 |
| body fat | 0.918 |
| whole milk | 1.030 |
| corn oil | 0.922 |
| mayonnaise | 0.910 |
| honey | 1.420 |
Example 1.3.2
The densities of some common liquids are in Table 1.3.2 Imagine you have five bottles containing colorless liquids (labeled A–E). You must identify them by measuring the density of each. Using a pipette, a laboratory instrument for accurately measuring and transferring liquids, you carefully measure 25.00 mL of each liquid into five beakers of known mass (1 mL = 1 cm3). You then weigh each sample on a laboratory balance. Use the tabulated data to calculate the density of each sample. Based solely on your results, can you unambiguously identify all five liquids?If necessary, review the use of significant figures in calculations in Essential Skills 1 (Section 1.8 "Essential Skills 1") prior to working this example.
Masses of samples: A, 17.72 g; B, 19.75 g; C, 24.91 g; D, 19.65 g; E, 27.80 g
Table 1.3.2 Densities of Liquids in Example 3
| Substance | Density at 25°C (g/cm3) |
|---|---|
| water | 0.998 |
| ethanol (the alcohol in beverages) | 0.789 |
| methanol (wood alcohol) | 0.792 |
| ethylene glycol (used in antifreeze) | 1.113 |
| diethyl ether (“ether”; once widely used as an anesthetic) | 0.708 |
| isopropanol (rubbing alcohol) | 0.785 |
Given: volume and mass
Asked for: density
Strategy:
A Calculate the density of each liquid from the volumes and masses given.
B Check to make sure that your answer makes sense.
C Compare each calculated density with those given in Table 1.3.2 . If the calculated density of a liquid is not significantly different from that of one of the liquids given in the table, then the unknown liquid is most likely the corresponding liquid.
D If none of the reported densities corresponds to the calculated density, then the liquid cannot be unambiguously identified.
Solution:
A Density is mass per unit volume and is usually reported in grams per cubic centimeter (or grams per milliliter because 1 mL = 1 cm3). The masses of the samples are given in grams, and the volume of all the samples is 25.00 mL (= 25.00 cm3). The density of each sample is calculated by dividing the mass by its volume (Equation 1.3.1). The density of sample A, for example, is
17.72g x 25.00 cm{3} = 0.7088 g/cm^{3}
Both the volume and the mass are given to four significant figures, so four significant figures are permitted in the result. (See Essential Skills 1, Section 1.8 "Essential Skills 1", for a discussion of significant figures.) The densities of the other samples (in grams per cubic centimeter) are as follows: B, 0.7900; C, 0.9964; D, 0.7860; and E, 1.112.
B Except for sample E, the calculated densities are slightly less than 1 g/cm3. This makes sense because the masses (in grams) of samples A–D are all slightly less than the volume of the samples, 25.00 mL. In contrast, the mass of sample E is slightly greater than 25 g, so its density must be somewhat greater than 1 g/cm3.
C Comparing these results with the data given in Table 1.3.2 shows that sample A is probably diethyl ether (0.708 g/cm3 and 0.7088 g/cm3 are not substantially different), sample C is probably water (0.998 g/cm3 in the table versus 0.9964 g/cm3 measured), and sample E is probably ethylene glycol (1.113 g/cm3 in the table versus 1.112 g/cm3 measured).
D Samples B and D are more difficult to identify for two reasons: (1) Both have similar densities (0.7900 and 0.7860 g/cm3), so they may or may not be chemically identical. (2) Within experimental error, the measured densities of B and D are indistinguishable from the densities of ethanol (0.789 g/cm3), methanol (0.792 g/cm3), and isopropanol (0.785 g/cm3). Thus some property other than density must be used to identify each sample.
Exercise
Given the volumes and masses of five samples of compounds used in blending gasoline, together with the densities of several chemically pure liquids, identify as many of the samples as possible.
| Sample | Volume (mL) | Mass (g) |
|---|---|---|
| A | 337 | 250.0 |
| B | 972 | 678.1 |
| C | 243 | 190.9 |
| D | 119 | 103.2 |
| E | 499 | 438.7 |
| Substance | Density (g/cm3) |
|---|---|
| benzene | 0.8787 |
| toluene | 0.8669 |
| m-xylene | 0.8684 |
| isooctane | 0.6979 |
| methyl t-butyl ether | 0.7405 |
| t-butyl alcohol | 0.7856 |
Answer: A, methyl t-butyl ether; B, isooctane; C, t-butyl alcohol; D, toluene or m-xylene; E, benzene
Summary
Matter is anything that occupies space and has mass. The three states of matter are solid, liquid, and gas. A physical change involves the conversion of a substance from one state of matter to another, without changing its chemical composition. Most matter consists of mixtures of pure substances, which can be homogeneous (uniform in composition) or heterogeneous (different regions possess different compositions and properties). Pure substances can be either chemical compounds or elements. Compounds can be broken down into elements by chemical reactions, but elements cannot be separated into simpler substances by chemical means. The properties of substances can be classified as either physical or chemical. Scientists can observe physical properties without changing the composition of the substance, whereas chemical properties describe the tendency of a substance to undergo chemical changes (chemical reactions) that change its chemical composition. Physical properties can be intensive or extensive. Intensive properties are the same for all samples; do not depend on sample size; and include, for example, color, physical state, and melting and boiling points. Extensive properties depend on the amount of material and include mass and volume. The ratio of two extensive properties, mass and volume, is an important intensive property called density.
Key Takeaway
- Matter can be classified according to physical and chemical properties.
Conceptual Problems
Please be sure you are familiar with the topics discussed in Essential Skills 1 (Section 1.8 "Essential Skills 1") before proceeding to the Conceptual Problems.
-
What is the difference between mass and weight? Is the mass of an object on Earth the same as the mass of the same object on Jupiter? Why or why not?
-
Is it accurate to say that a substance with a mass of 1 kg weighs 2.2 lb? Why or why not?
-
What factor must be considered when reporting the weight of an object as opposed to its mass?
-
Construct a table with the headings “Solid,” “Liquid,” and “Gas.” For any given substance, state what you expect for each of the following:
- the relative densities of the three phases
- the physical shapes of the three phases
- the volumes for the same mass of compound
- the sensitivity of the volume of each phase to changes in temperature
- the sensitivity of the volume to changes in pressure
-
Classify each substance as homogeneous or heterogeneous and explain your reasoning.
- platinum
- a carbonated beverage
- bronze
- wood
- natural gas
- Styrofoam
-
Classify each substance as homogeneous or heterogeneous and explain your reasoning.
- snowflakes
- gasoline
- black tea
- plastic wrap
- blood
- water containing ice cubes
-
Classify each substance as a pure substance or a mixture and explain your reasoning.
- seawater
- coffee
- 14-karat gold
- diamond
- distilled water
-
Classify each substance as a pure substance or a mixture.
- cardboard
- caffeine
- tin
- a vitamin tablet
- helium gas
-
Classify each substance as an element or a compound.
- sugar
- silver
- rust
- rubbing alcohol
- copper
-
Classify each substance as an element or a compound.
- water
- iron
- hydrogen gas
- glass
- nylon
-
What techniques could be used to separate each of the following?
- sugar and water from an aqueous solution of sugar
- a mixture of sugar and sand
- a heterogeneous mixture of solids with different solubilities
-
What techniques could be used to separate each of the following?
- solid calcium chloride from a solution of calcium chloride in water
- the components of a solution of vinegar in water
- particulates from water in a fish tank
-
Match each separation technique in (a) with the physical/chemical property that each takes advantage of in (b).
- crystallization, distillation, filtration
- volatility, physical state, solubility
-
Classify each statement as an extensive property or an intensive property.
- Carbon, in the form of diamond, is one of the hardest known materials.
- A sample of crystalline silicon, a grayish solid, has a mass of 14.3 g.
- Germanium has a density of 5.32 g/cm3.
- Gray tin converts to white tin at 13.2°C.
- Lead is a bluish-white metal.
-
Classify each statement as a physical property or a chemical property.
- Fluorine etches glass.
- Chlorine interacts with moisture in the lungs to produce a respiratory irritant.
- Bromine is a reddish-brown liquid.
- Iodine has a density of 11.27 g/L at 0°C.
Numerical Problems
Please be sure you are familiar with the topics discussed in Essential Skills 1 (Section 1.8 "Essential Skills 1") before proceeding to the Numerical Problems.
-
If a person weighs 176 lb on Earth, what is his or her mass on Mars, where the force of gravity is 37% of that on Earth?
-
If a person weighs 135 lb on Earth, what is his or her mass on Jupiter, where the force of gravity is 236% of that on Earth?
-
Calculate the volume of 10.00 g of each element and then arrange the elements in order of decreasing volume. The numbers in parentheses are densities.
- copper (8.92 g/cm3)
- calcium (1.54 g/cm3)
- titanium (4.51 g/cm3)
- iridium (22.85 g/cm3)
-
Given 15.00 g of each element, calculate the volume of each and then arrange the elements in order of increasing volume. The numbers in parentheses are densities.
- gold (19.32 g/cm3)
- lead (11.34 g/cm3)
- iron (7.87 g/cm3)
- sulfur (2.07 g/cm3)
-
A silver bar has dimensions of 10.00 cm × 4.00 cm × 1.50 cm, and the density of silver is 10.49 g/cm3. What is the mass of the bar?
-
Platinum has a density of 21.45 g/cm3. What is the mass of a platinum bar measuring 3.00 cm × 1.50 cm × 0.500 cm?
-
Complete the following table.
Density (g/cm3) Mass (g) Volume (cm3) Element 3.14 79.904 Br 3.51 3.42 C 39.1 45.5 K 11.34 207.2 Pb 107.868 10.28 Ag 6.51 14.0 Zr -
Gold has a density of 19.30 g/cm3. If a person who weighs 85.00 kg (1 kg = 1000 g) were given his or her weight in gold, what volume (in cm3) would the gold occupy? Are we justified in using the SI unit of mass for the person’s weight in this case?
-
An irregularly shaped piece of magnesium with a mass of 11.81 g was dropped into a graduated cylinder partially filled with water. The magnesium displaced 6.80 mL of water. What is the density of magnesium?
-
The density of copper is 8.92 g/cm3. If a 10.00 g sample is placed in a graduated cylinder that contains 15.0 mL of water, what is the total volume that would be occupied?
-
At 20°C, the density of fresh water is 0.9982 kg/m3, and the density of seawater is 1.025 kg/m3. Will a ship float higher in fresh water or in seawater? Explain your reasoning.
Answers
-
Unlike weight, mass does not depend on location. The mass of the person is therefore the same on Earth and Mars: 176 lb ÷ 2.2 lb/kg = 80 kg.
-
-
- Cu: 1.12 cm3
- Ca: 6.49 cm3
- Ti: 2.22 cm3
- Ir: 0.4376 cm3
Volume decreases: Ca > Ti > Cu > Ir
-
-
629 g
-
-
-
-
1.74 g/cm3
Contributors
- Anonymous
Modified by Joshua Halpern (Howard University)
Videos:
Milk Under a Microscope from JDH Microscopy @ YouTube
Laboratory Distillation from Sci Vis Lab @ YouTube
Perfume Distillation from Essential Oil Distillation by Strawberry Banke @ YouTube
Crystallization of Sodium Acetate from Mike Shin @ YouTube
Electrolysis of Water from Robert Raves's @ YouTube
Applet
Density Applet from PhET