5.5: Conceptual Analysis of States of Matter and Phase Changes as a Function of Temperature
- Page ID
- 213146
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Given the melting and boiling points of a substance, identify the states of matter that exist and the phases changes that occur for that substance across a range of temperatures.
The previous three sections of this chapter have discussed temperature conversions, states of matter, and phase changes, respectively. These concepts can be integrated with one another through a conceptual analysis of the states of matter that exist and the phase changes that occur for a substance across a range of temperatures.
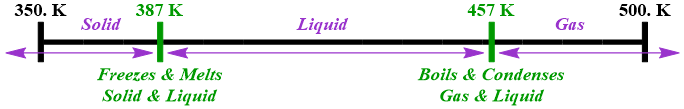
For example, consider molecular iodine, which melts at 387 Kelvin and boils at 457 Kelvin. Identify the states of matter that exist and the phases changes that occur for molecular iodine between 350. Kelvin and 500. Kelvin.
As stated in the previous section, a substance that is present at a temperature below its freezing point will exist exclusively in the solid state of matter. If the temperature exceeds the boiling point of a substance, that chemical will be present only in the gaseous state of matter. Finally, a substance will exist only as a liquid in between these temperatures. Furthermore, complementary phase changes, which involve conversions between the same two states of matter, must both occur at the same singular temperature. Finally, both of the states of matter that are associated with those transformations must exist at that phase change temperature.
Based on the given information, molecular iodine exists solely in the solid state of matter at temperatures below 387 Kelvin. Between 387 Kelvin and 457 Kelvin, this chemical will only be present in the liquid state of matter. At temperatures exceeding 457 Kelvin, this substance will exist exclusively in the gaseous state of matter. Furthermore, freezing and melting are complementary phase changes, as are boiling and condensation. Therefore, molecular iodine both freezes and melts at 387 Kelvin and, consequently, is present in both the solid and liquid states of matter at this temperature. Finally, this chemical both boils and condenses at 457 Kelvin and, therefore, exists in both the gaseous and liquid states of matter at this temperature.
This information can also be represented visually, as shown below in Figure \(\PageIndex{1}\).
Consider phosphorus, which melts at 112 degrees Fahrenheit and boils at 537 degrees Fahrenheit. Identify the states of matter that exist and the phases changes that occur for phosphorus between 50. degrees Fahrenheit and 650. degrees Fahrenheit.
- Answer
- A substance that is present at a temperature below its freezing point will exist exclusively in the solid state of matter. If the temperature exceeds the boiling point of a substance, that chemical will be present only in the gaseous state of matter. Finally, a substance will exist only as a liquid in between these temperatures. Furthermore, complementary phase changes, which involve conversions between the same two states of matter, must both occur at the same singular temperature. Finally, both of the states of matter that are associated with those transformations must exist at that phase change temperature.
Based on the given information, phosphorus exists solely in the solid state of matter at temperatures below 112 degrees Fahrenheit. Between 112 degrees Fahrenheit and 537 degrees Fahrenheit, this chemical will only be present in the liquid state of matter. At temperatures exceeding 537 degrees Fahrenheit, this substance will exist exclusively in the gaseous state of matter. Furthermore, freezing and melting are complementary phase changes, as are boiling and condensation. Therefore, phosphorus both freezes and melts at 112 degrees Fahrenheit and, consequently, is present in both the solid and liquid states of matter at this temperature. Finally, this chemical both boils and condenses at 537 degrees Fahrenheit and, therefore, exists in both the gaseous and liquid states of matter at this temperature.
This information can also be represented visually, as shown below in Figure \(\PageIndex{2}\).
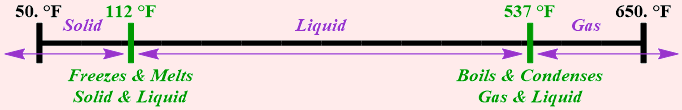
Figure \(\PageIndex{2}\): A summary of the states of matter that exist and the phases changes that occur for phosphorus between 50. degrees Fahrenheit and 650. degrees Fahrenheit.

