3.5: Thiols
- Page ID
- 338694
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Learning Objectives
- Identify thiols by the presence of an SH group.
- Identify thioethers by the presence of an R–S–R′ group
Because sulfur is in the same group (6A) of the periodic table as oxygen, the two elements have some similar properties. We might expect sulfur to form organic compounds related to those of oxygen, and indeed it does. Thiols (also called mercaptans) are organic molecules that contain a sulfhydryl (–SH) group. These compounds, which are sulfur analogs of alcohols, have the general formula R–SH. Methanethiol (also called methyl mercaptan), has the formula CH3SH. Ethanethiol (ethyl mercaptan) is the most common odorant for liquid propane (LP) gas.
The mild oxidation of thiols gives compounds called disulfides.
\[\mathrm{2 RSH \xrightarrow{\,[O]\,} RSSR} \]
The amino acids cysteine [HSCH2CH(NH2)COOH] and methionine [CH3SCH2CH2CH(NH2)COOH] (Figure \(\PageIndex{1}\)) contain sulfur atoms, as do all proteins that contain these amino acids. Disulfide linkages (–S–S–) between protein chains are extremely important in protein structure.
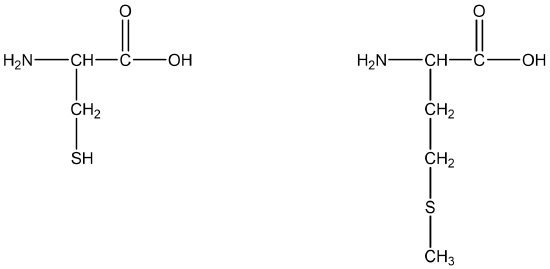
Thioethers, which are sulfur analogs of ethers, have the form general formula R–S–R′. An example is dimethylsulfide (CH3–S–CH3), which is responsible for the sometimes unpleasant odor of cooking cabbage and related vegetables. Note that methionine has a thioether functional group.
Career Focus: Paramedic
Paramedics are highly trained experts at providing emergency medical treatment. Their critical duties often include rescue work and emergency medical procedures in a wide variety of settings, sometimes under extremely harsh and difficult conditions. Like other science-based professions, their work requires knowledge, ingenuity, and complex thinking, as well as a great deal of technical skill. The recommended courses for preparation in this field include anatomy, physiology, medical terminology, and—not surprisingly—chemistry. An understanding of basic principles of organic chemistry, for example, is useful when paramedics have to deal with such traumas as burns from fuel (hydrocarbons) or solvent (alcohols, ethers, esters, and so on) fires and alcohol and drug overdoses.
To become a paramedic requires 2–4 y of training and usually includes a stint as an emergency medical technician (EMT). An EMT provides basic care, can administer certain medications and treatments, such as oxygen for respiratory problems and epinephrine (adrenalin) for allergic reactions, and has some knowledge of common medical conditions. A paramedic, in contrast, must have extensive knowledge of common medical problems and be trained to administer a wide variety of emergency drugs.
Paramedics usually work under the direction of a medical doctor with a title such as “medical director.” Some paramedics are employed by fire departments and may work from a fire engine that carries medical equipment as well as fire-fighting gear. Some work from hospital-sponsored ambulances and continue to care for their patients after reaching the hospital emergency room. Still other paramedics work for a government department responsible for emergency health care in a specific geographical area. Finally, some work for private companies that contract to provide service for a government body.
An experienced paramedic has a broad range of employment options, including training for mountain or ocean rescue, working with police department special weapons and tactics (SWAT) teams, or working in isolated settings such as on oil rigs. With their expertise at treating and stabilizing patients before quickly moving them to a hospital, paramedics often provide the first critical steps in saving an endangered life. The following quotation, inscribed on the Arlington National Cemetery headstone of Army Lieutenant R. Adams Cowley, who is often called the “father” of shock trauma medicine, serves as the motto for many paramedic units: “Next to creating a life the finest thing a man can do is save one.” —Abraham Lincoln
Naming Thiols
Rules for naming thiols are similar to rules for naming alcohols. Thiols are named by adding the word –thiol as the suffix of the parent name. The longest
carbon chain is then numbered to give the sulfhydryl group the lowest possible number.
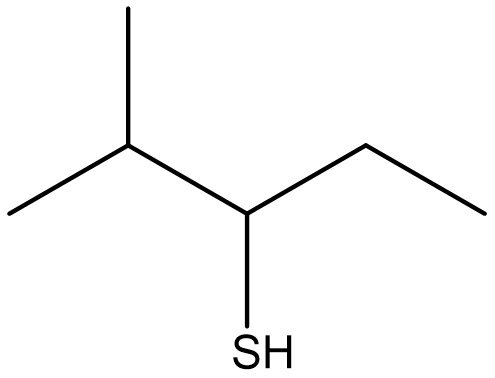
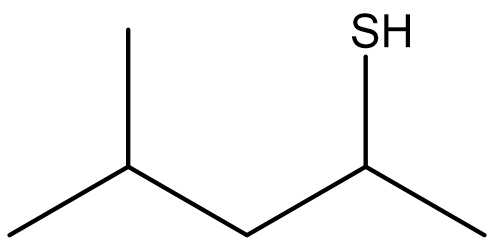
Based on the rules for naming thiols, the molecule shown in Figure \(\PageIndex{2}\) would be named 2-methyl-3-pentanethiol (or 2-methylpentane-3-thiol). Since the functional group has priority in numbering, if the sulhydryl group is bonded to a ring, the carbon that it is bonded to is assigned to C1 and the number is omitted from the name.
Exercise \(\PageIndex{1}\)
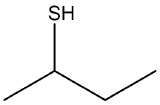
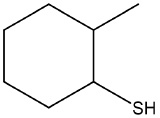
Name the following compounds.
Answer
- The longest continuous chain of carbon has four carbon atoms, so the stem name is butane. The parent name is obtained by adding the word thiol, to give butanethiol. We number from the left to give the sulfhydryl group the lowest number. Since there are no substituents present, the name of the molecule is 2-butanethiol (or butane-2-thiol).
- The longest continuous chain of carbon has six carbon atoms in a ring, so the stem name is cyclohexane. The parent name is obtained by adding the word thiol, to give cyclohexanethiol. The carbon that has the sulfhydryl group is assigned as C1, since any carbon in the ring can be C1. The ring is then number counterclockwise to give the methyl substituent the lowest possible number. Therefore, the the name of the molecule is 2-methylcyclohexanethiol.
Exercise \(\PageIndex{1}\)
Name the following compounds.
Summary
- Thiols, thioethers, and disulfides are common in biological compounds that contain carbon.
- Nomenclature of thiols: location and identity of substituents + parent alkane + thiol suffix (with location of functional group)