15.5.1: Basic Concepts in Membranes
- Page ID
- 306711
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Source: BiochemFFA_3_1.pdf. The entire textbook is available for free from the authors at http://biochem.science.oregonstate.edu/content/biochemistry-free-and-easy
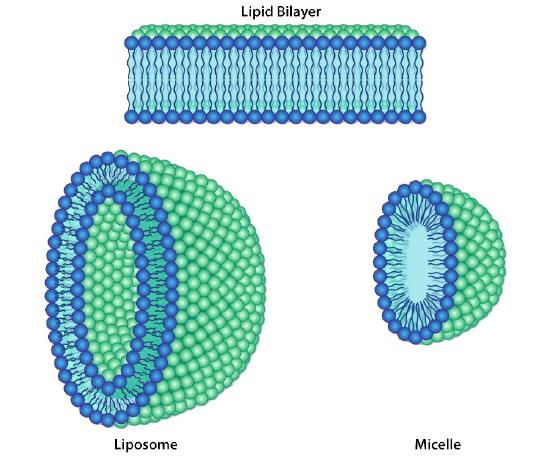
Lipid bilayers
The protective membrane around cells contains many components, including cholesterol, proteins, glycolipids, glycerophospholipids, and sphingolipids. The last two of these will, when mixed vigorously with water, spontaneously form what is called a lipid bilayer (Figure 3.1), which serves as a protective boundary for the cell that is largely impermeable to the movement of most materials across it. With the notable exceptions of water, carbon dioxide, carbon monoxide, and oxygen, most polar/ionic require transport proteins to help them to efficiently navigate across the bilayer. The orderly movement of these compounds is critical for the cell to be able to 1) get food for energy; 2) export materials; 3) maintain osmotic balance; 4) create gradients for secondary transport; 5) provide electromotive force for nerve signaling; and 6) store energy in electrochemical gradients for ATP production (oxidative phosphorylation or photosynthesis). In some cases, energy is required to move the substances (active transport).
Facilitated Diffusion
In other cases, no external energy is required and they move by diffusion through specific cellular channels. This is referred to as facilitated diffusion. Before we discuss movement of materials across membranes, it is appropriate we discuss the composition of cellular membranes. Plasma membranes differ from cell walls both in the materials comprising them and in their flexibility. Cell walls will be covered near the end of this chapter.
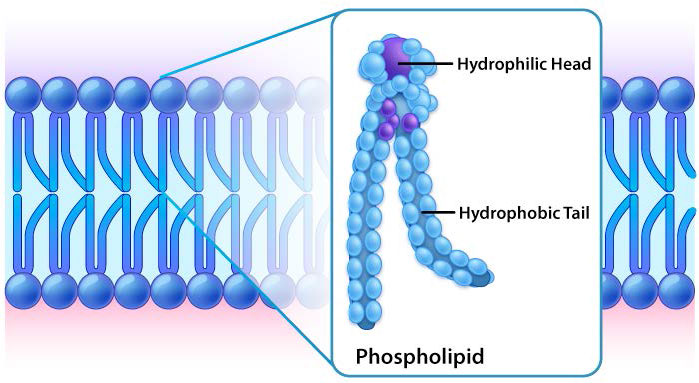
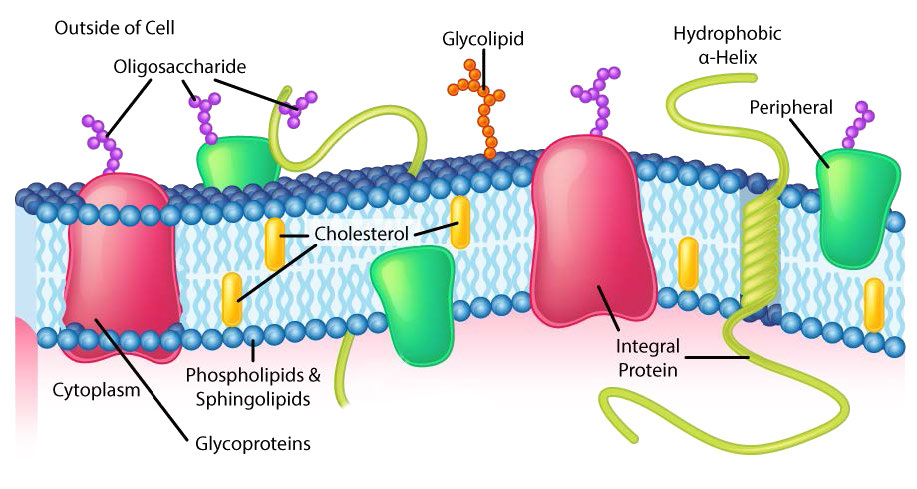
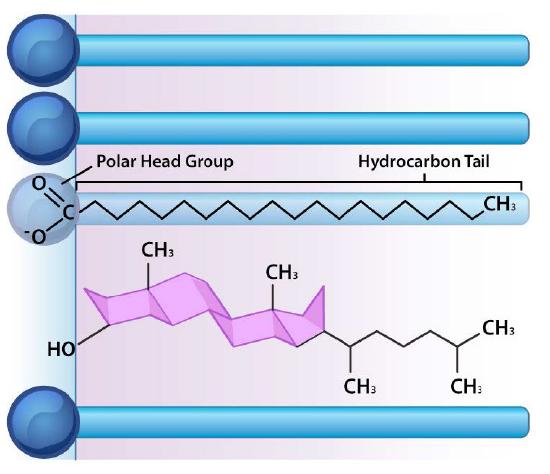
Though some cells do not have cell walls (animal cells) and others do (bacteria, fungi, and plants), there is commonality among cells in that they all possess plasma membranes. There is also commonality in the components of the membranes, though the relative amount of constituents varies. Figures 3.1 and 3.2 illustrate the structure and environments of plasma membranes. All plasma membranes contain a significant amount of amphiphilic substances linked to fatty acids. These include the glycerophospholipids and the sphingolipids. The fatty acid(s) are labeled as hydrophobic tails in the figures.
Hydrophilic heads
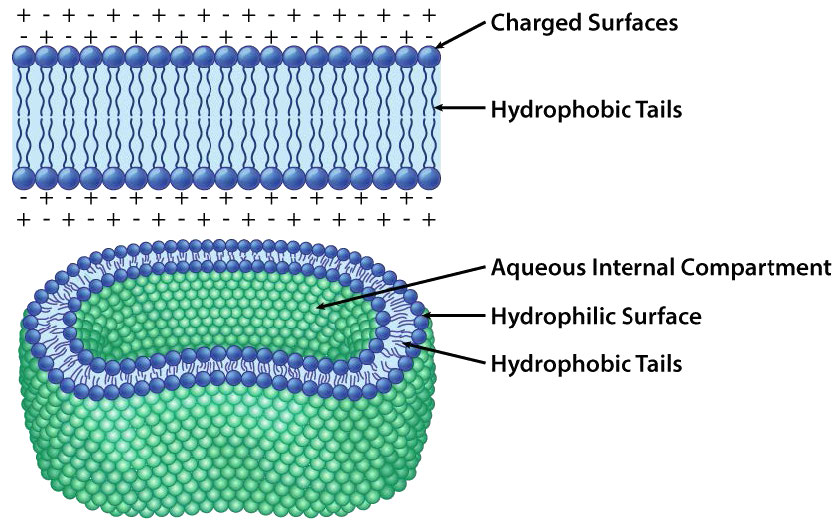
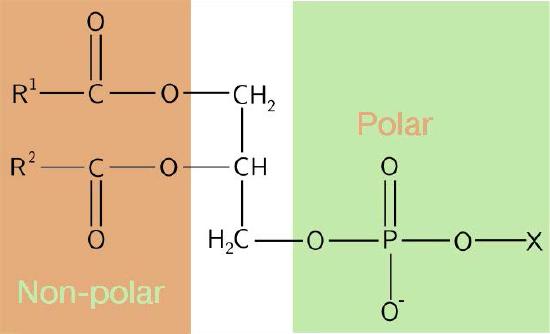
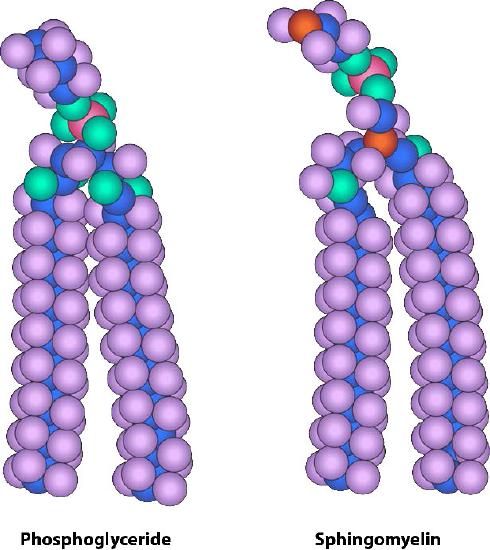
The composition of the hydrophilic heads varies considerably. In glycerophospholipids, a phosphate is always present, of course, and it is often esterified to another substance to make a phosphatide (Figure 3.3). Common compounds linked to the phosphate (at the X position) include serine, ethanolamine, and choline. These vary in the their charges so in this way, the charge on the external or internal surface can be controlled. Cells tend to have more negative charges on the exterior half of the lipid bilayer (called the outer leaflet) and more positive charges on the interior half (inner leaflet).
Sphingolipids
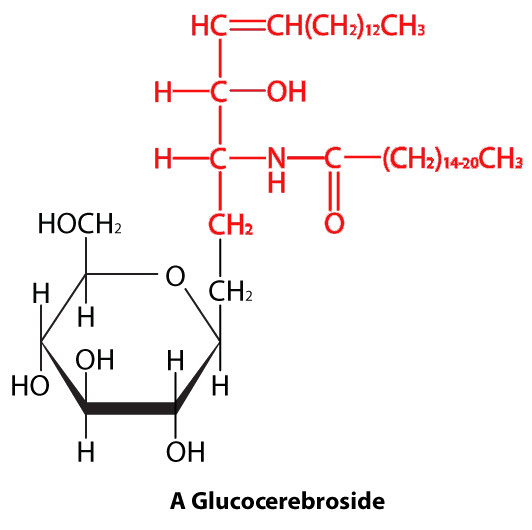
In sphingolipids (Figure 3.4), the hydrophilic head can contain a phosphate linked to ethanolamine or choline and this describes the structure of sphingomyelin, an important component of neural membranes. Most sphingolipids lack the phosphate and have instead a hydrophilic head of a single sugar (cerebrosides) or a complex oligosaccharide (gangliosides).
Water exclusion
In each case, the glycerophospholipid or sphingolipid has one end that is polar and one end that is non-polar. As we saw in the organization of amino acids with hydrophobic side chains occurring preferentially on the inside of a folded protein to exclude water, so too do the non-polar portions of these amphiphilic molecules arrange themselves so as to exclude water. Remember that the cytoplasm of a cell is mostly water and the exterior of the cell is usually bathed in an aqueous layer. It therefore makes perfect sense that the polar portions of the membrane molecules arrange themselves as they do - polar parts outside interacting with water and non-polar parts in the middle of the bilayer avoiding/excluding water.
Other components of lipid bilayer
Besides glycerophospholipids and sphingolipids, there are other materials commonly found in lipid bilayers of cellular membranes. Two important prominent ones are cholesterol (Figure 3.13) and proteins. Besides serving as a metabolic precursor of steroid hormones and the bile acids, cholesterol’s main role in cells is in the membranes. The flatness and hydrophobicity of the sterol rings allow cholesterol to interact with the nonpolar portions of the lipid bilayer while the hydroxyl group on the end can interact with the hydrophilic part.
Membrane fluidity
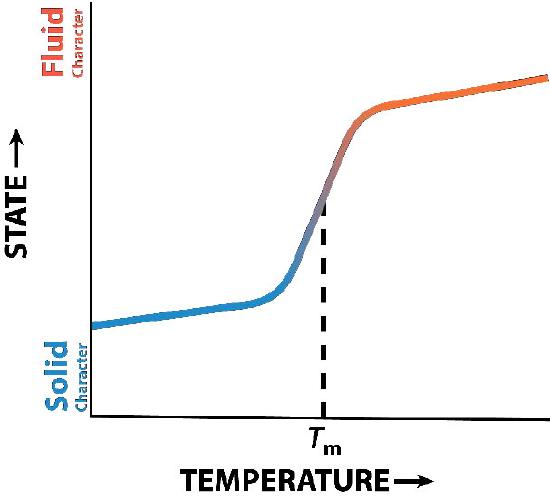
Cholesterol’s function in the lipid bilayer is complex (Figure 3.13). It influences membrane fluidity. Figure 3.14 shows the phase transition for a membrane as it is heated, moving from a more “frozen” character to that of a more “fluid” one as the temperature rises. The mid-point of this transition, referred to as the Tm, is influenced by the fatty acid composition of the lipid bilayer compounds. Longer and more saturated fatty acids will favor higher Tm values, whereas unsaturation and short fatty acids will favor lower Tm values. It is for this reason that fish, which live in cool environments, have a higher level of unsaturated fatty acids in them - to use to make membrane lipids that will remain fluid at ocean temperatures. Interestingly, cholesterol does not change the Tm value, but instead widens the transition range between frozen and fluid forms of the membrane, allowing it to have a wider range of fluidity.
Barrier
Transport of materials across membranes is essential for a cell to exist. The lipid bilayer is an effective barrier to the entry of most molecules and without a means of allowing food molecules to enter a cell, it would die. The primary molecules that move freely across the lipid bilayer are small, uncharged ones, such as H2O, CO2, CO, and O2, so larger molecules, like glucose, that the cell needs for energy, would be effectively excluded if there were not proteins to facilitate its movement across the membrane.
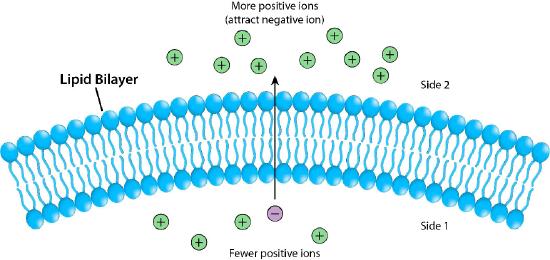
Figure 3.17 depicts the barrier that the lipid bilayer provides to movement across it and the pressures (ionic attraction, in this case) that can affect movement. Potential energy from charge and concentration differences are harvested by cells for purposes that include synthesis of ATP, and moving materials against a concentration gradient in a process called active transport.
Membrane proteins
Proteins in a lipid bilayer can vary in quantity enormously, depending on the membrane. Protein content by weight of various membranes typically ranges between 30 and 75% by weight. Some mitochondrial membranes can have up to 90% protein. Proteins linked to and associated with membranes come in several types.
Transmembrane proteins
Transmembrane proteins are integral membrane proteins that completely span from one side of a biological membrane to the other and are firmly embedded in the membrane (Figure 3.18). Transmembrane proteins can function as docking sites for attachment (to the extracellular matrix, for example), as receptors in the cellular signaling system, or facilitate the specific transport of molecules into or out of the cell.
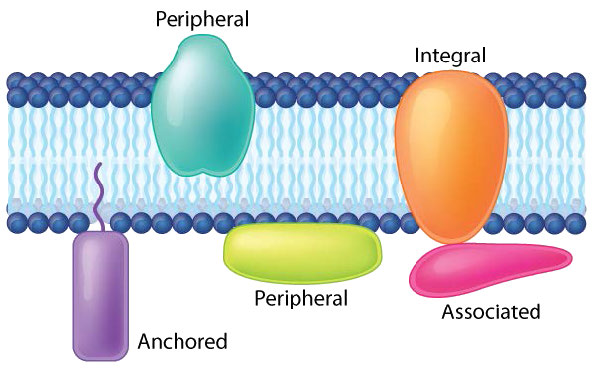
Example of integrated/ transmembrane proteins include those involved in transport (e.g., Na+/K+ ATPase), ion channels (e.g., potassium channel of nerve cells) and signal transduction across the lipid bilayer (e.g., GProtein Coupled Receptors).
Peripheral membrane proteins interact with part of the bilayer (usually does not involve hydrophobic interactions), but do not project through it. A good example is phospholipase A2, which cleaves fatty acids from glycerophospholipids in membranes. Associated membrane proteins typically do not have external hydrophobic regions, so they cannot embed in a portion of the lipid bilayer, but are found near them. Such association may arise as a result of interaction with other proteins or molecules in the lipid bilayer. A good example is ribonuclease.


