4.4: HPLC Components at Duke
- Page ID
- 401518
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \) \( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)\(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\) \(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\)\(\newcommand{\AA}{\unicode[.8,0]{x212B}}\)
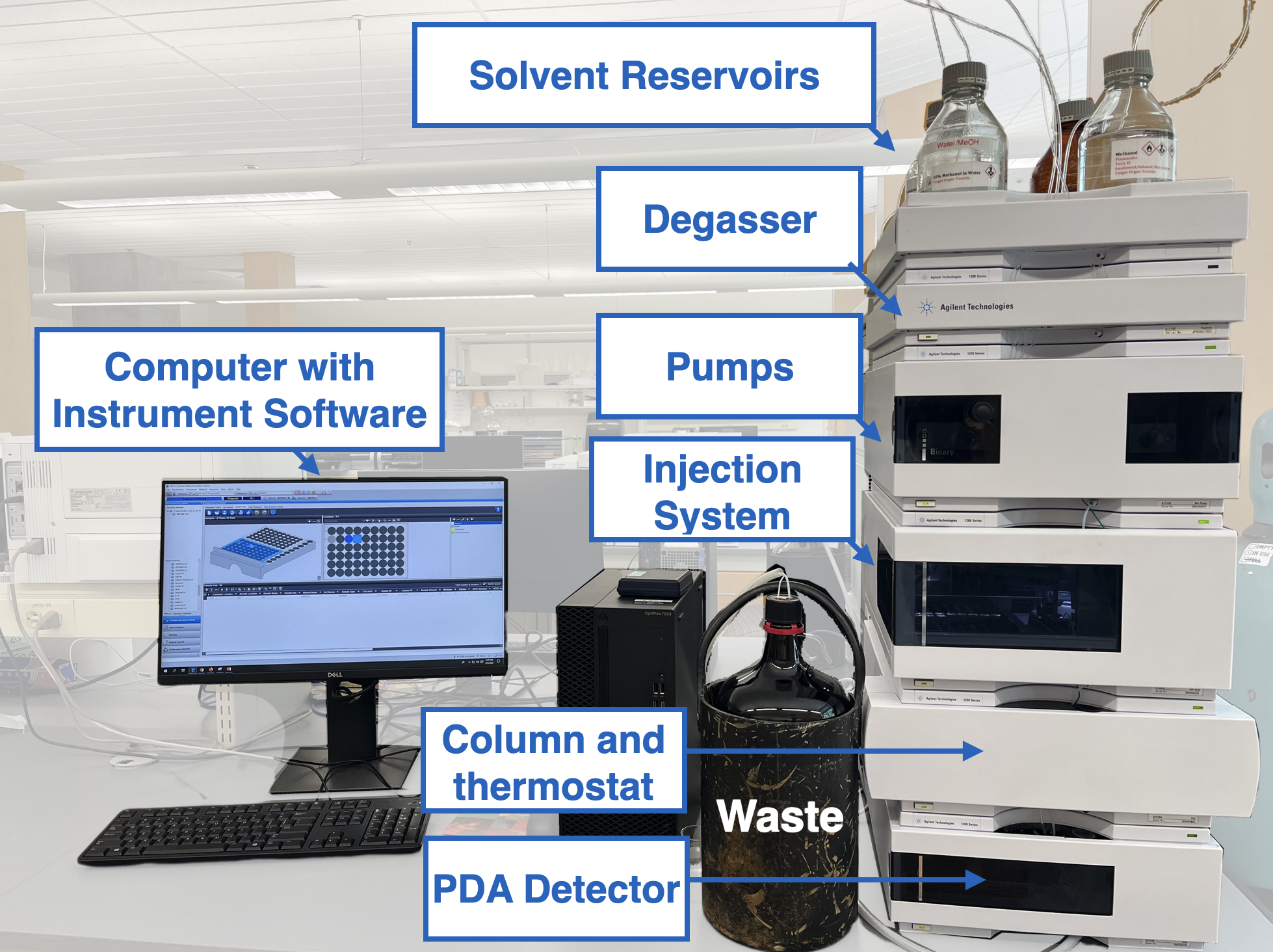
Duke Chem undergraduate teaching labs use Agilent 1000 series high performance liquid chromatographic systems. Important details of the components you will use are listed below in order of their position on the instrument (Figure \(\PageIndex{1}\)) from top to bottom as you view the instrument.
- MOBILE PHASE RESERVOIRS: There are four 1-liter gas-tight glass solvent reservoir bottles labeled A1, A2, B1, and B2. Reservoir A1 contains deionized water. Reservoir B2 contains water with 10% acetonitrile. Reservoir B1 contains methanol, and reservoir A2 contains acetonitrile. All solvents have been filtered through 0.45µ filters prior to introduction to the reservoirs. The solvents are degassed by a vacuum degasser (Agilent 1379B) that sits directly under the solvent reservoirs.
- PUMP (AGILENT G1312A): Our instrument uses a binary pump system. The Binary pump comprises two identical pumps integrated into one housing. Solvents from bottles A1 and A2 are assigned to one pump, while those from B1 and B2 are assigned to the other pump. Binary solvent mixtures are accomplished by proportional passage of solvent from each pump. The mixed solvent, or mobile phase, is then pumped through the column. Mobile phase changes can be accomplished easily and quickly, which is especially useful for re-equilibration following a gradient run.
- AUTOMATED SAMPLE INJECTION SYSTEM (AGILENT G1367A): This instrument uses a programmable and automated sample injection system. Samples for analysis are placed in 2 mL glass vials and sealed with a cap. The vials are placed at fixed locations in a 100-vial tray. A syringe system is used to withdraw a controlled volume of sample from the vial chosen for analysis. This sample is transferred to the sample loop of the injection valve. Rotation of the injection valve results in the sample loop being inserted in the flow path of the mobile phase just before it enters the head of the column. In this instrument, the sample volume is determined by the volume withdrawn by the needle up to a maximum of 100 µL.
- COLUMN: The 10 cm x 4.6 mm i.d. stainless steel chromatographic column is packed with a homogeneous bed of uniform spherical silica gel particles which are 5 microns in diameter with a pore size of 120 Å. Some of the silanol (SiOH) groups on the surface of each particle have been derivatized with an eighteen-carbon alkane (C18H37) chain that gives the surface a certain degree of hydrophobic character. Octadecyl modified silica is one of the most common stationary phases used in reverse-phase LC. Chemical modification of the surface is preferred to coating by adsorption, because the latter surface may be removed by dissolution in the mobile phase. Separation in LC is achieved by means of differences in the interactions of the analytes with both the mobile and stationary phases. In this experiment, you will perform reverse-phase LC using a nonpolar stationary phase (C18) and a polar mobile phase.
- DIODE ARRAY DETECTOR (AGILENT G1315B): Methods of detection used for HPLC include UV absorption, IR absorption, fluorescence, refractive index, electrical conductivity, mass spectrometry, electrochemical and radiochemical. Many modern LC instruments use diode array UV detectors. As well as the traditional two-dimensional presentation of detector signal against time, it is possible to display the UV spectra of the components giving rise to different chromatographic peaks. Differences in the spectrum between the front and tailing edges of a peak can be used to detect unresolved impurities. Such spectra can be captured in 'real time' without the need to interrupt the eluent flow. It is possible to construct pseudo-three-dimensional, or isometric plots, of absorbance, wavelength and time. (Note: This type of plot can be generated using the computer simulation used in your pre-lab assignment. You are encouraged to investigate this.) The Agilent instrument uses a diode array UV detector with a deuterium lamp source. All wavelengths in the range 190-950 nm are monitored simultaneously during elution. The detector can monitor an entire wavelength range or up to five discrete pre-selected wavelengths. To minimize the size of data files, complete spectrum scans are only saved when needed; typically monitoring at one or more select wavelengths is sufficient for most common analytical work. By careful selection of the wavelength(s) used for detection, the response of a particular analyte can be enhanced at the expense of possible interferences.
- COMPUTER: A desktop computer is interfaced to each LC module through an Ethernet interface. Instrument control, data acquisition and storage, and chromatographic peak integration are all initiated from the computer.