12.1: Brønsted–Lowry Acids and Bases
- Page ID
- 195154
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Learning Objectives
- Identify acids, bases, and conjugate acid-base pairs according to the Brønsted-Lowry definition
- Write equations for acid and base ionization reactions
- Describe the acid-base behavior of amphiprotic substances
- Understand acid-base reactivity as a proton transfer process.
In previous chapters, we have understood acids and bases in terms of the Arrhenius definition: an acid is a compound that dissolves in water to yield hydronium ions (\(H_3O^+\)) and a base is a compound that dissolves in water to yield hydroxide ions (\(\ce{OH-}\)). This definition is not wrong; it is simply limited. In this chapter, we will extend the definition of an acid or a base using the more general definition proposed in 1923 by the Danish chemist Johannes Brønsted and the English chemist Thomas Lowry. Their definition centers on the proton, \(\ce{H^+}\). A proton is what remains when a normal hydrogen atom, \(\ce{^1_1H}\), loses an electron. A compound that donates a proton to another compound is called a Brønsted-Lowry acid, and a compound that accepts a proton is called a Brønsted-Lowry base.
| Definition | Acid | Base |
|---|---|---|
| Arrhenius | \(H^+\) donor | \(OH^−\) donor |
| Brønsted–Lowry | \(H^+\) donor | \(H^+\) acceptor |
Brønsted-Lowry Acids
Brønsted-Lowry acids may be neutral molecules such as \(\ce{HCl}\), \(\ce{H_2SO_4}\), or acetic acid (\(\ce{CH_3COOH}\)). Anions (such as \(\ce{HSO_4^-}\), \(\ce{H_2PO_4^-}\), \(\ce{HS^-}\), and \(\ce{HCO_3^-}\)) and cations (such as \(\ce{H_3O^+}\), \(\ce{NH_4^+}\), and \(\ce{[Al(H_2O)_6]^{3+}}\)) may also act as acids. In general, we can symbolize any acid as \(\ce{HA}\) and describe the donation of a proton to a water molecule as follows:
\[\ce{HA + H_2O \rightleftharpoons H_3O^+ + A^-}\]
We call the product, \(\ce{A^-}\), that remains after an acid donates a proton the conjugate base of the acid. In the generalized example above, the anion (\(\ce{A^-}\)) is the conjugate base of the acid (\(\ce{HA}\)). This species is called a base because it can accept a proton (to re-form the original acid).
The reaction between a Brønsted-Lowry acid and water is called acid ionization. For example, when hydrogen fluoride dissolves in water and ionizes, protons are donated from hydrogen fluoride molecules to water molecules, yielding hydronium ions and fluoride ions:
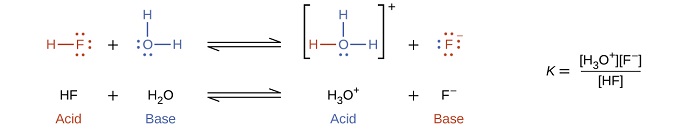
The fluoride ion (\(\ce{F^-}\)) is the conjugate base of hydrofluoric acid (\(\ce{HF}\)). Here are several other examples of acid ionization in water:
\[\ce{H_2SO_4 + H_2O \rightleftharpoons H_3O^+ + HSO_4^{−}}\]
\[\ce{NH_4^+ + H_2O \rightleftharpoons H_3O^+ + NH_3} \]
In each case, the acid ionization process results in the formation of the conjugate base! Identifying acid-base conjugate pairs will help you understand acid-base reactivity.
Example \(\PageIndex{1}\): Conjugate Acid-Base Pairs
Identify the conjugate base for each of the following acids:
a) acetic acid (\(\ce{HC_2H_3O_2}\))
b) hydrocyanic acid (\(\ce{HCN}\))
c) phosphoric acid (\(\ce{H_3PO_4}\))
Solution
a) \(\ce{C_2H_3O_2^-}\)
b) \(\ce{CN^-}\)
c) \(\ce{H_2PO_4^{-}}\)
Brønsted-Lowry Bases
Brønsted-Lowry bases may, likewise, be neutral molecules (such as \(\ce{NH_3}\) and \(\ce{CH_3NH_2}\)), anions (such as \(\ce{OH^-}\), \(\ce{HS^-}\), \(\ce{HCO_3^-}\), \(\ce{CO_3^{2−}}\), \(\ce{F^-}\), and \(\ce{PO_4^{3−}}\)), or cations (such as \(\ce{[Al(H_2O)_5OH]^{2+}}\)). The most familiar bases are ionic compounds such as \(\ce{NaOH}\) and \(\ce{Ca(OH)_2}\), which contain the hydroxide ion, \(\ce{OH^-}\).
When we add a base to water, a base ionization reaction occurs in which a proton is transferred from a water molecule and accepted by the base molecule (or ion). For example, adding ammonia to water yields hydroxide ions and ammonium ions. We call the product that results when a base accepts a proton the base’s conjugate acid. This species is an acid because it can give up a proton (and thus re-form the base).
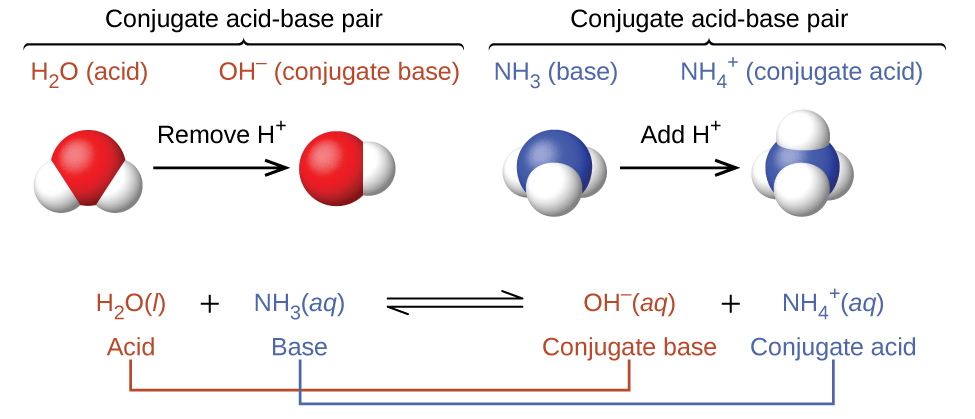
Notice that both these ionization reactions are represented as equilibrium processes. The relative extent to which these acid and base ionization reactions proceed is an important topic treated in a later section of this chapter.
Acid-Base Reactions
Acid-base reactivity under the Brønsted-Lowry definition can be considered a proton transfer process. In this process the proton is transferred from the acid to the base. Consider the following reaction between hydrocyanic acid (\(\ce{HCN}\)) and ammonia (\(\ce{NH_3}\)):
Example \(\PageIndex{2}\): Acid-Base Reaction (Proton Transfer)
Write and equation for the reaction that would occur between hydrocyanic acid (\(\ce{HCN}\)) and ammonia (\(\ce{NH_3}\)).
Solution
This is a proton transfer reaction. The acid will donate a proton and the base will accept a proton.
\(\ce{HCN(aq)+ NH_3(aq) \rightleftharpoons CN^-(aq) + NH_4^+ (aq)}\)
Amphiprotic Species
Many molecules and ions may either gain or lose a proton under the appropriate conditions. Such species are said to be amphiprotic. Another term used to describe such species is amphoteric, which is a more general term for a species that may act either as an acid or a base by any definition (not just the Brønsted-Lowry one). Consider for example the bicarbonate ion, which may either donate or accept a proton as shown here:
\[\ce{HCO_3^- (aq) + H_2O (l) \rightleftharpoons CO_3^{2-} (aq) + H_3O^+ (aq)}\]
\[\ce{HCO_3^- (aq) + H_2O (l) \rightleftharpoons H_2CO_3 (aq) + OH^- (aq)}\]
Example \(\PageIndex{3}\): The Acid-Base Behavior of an AmphoPROTIC Substance
Write separate equations representing the ionization in water of \(\ce{HSO3-}\)
- as an acid
- as a base
Solution
- \(\ce{HSO_3^- (aq) + H_2O (aq) \rightleftharpoons SO_3^{2-}(aq) + H_3O^+ (aq)}\)
- \(\ce{HSO_3^- (aq) + H_2O (aq) \rightleftharpoons H_2SO_3(aq)+ OH^-(aq)}\)
Exercise \(\PageIndex{1}\)
Write separate equations representing the reaction of \(\ce{H2PO4-}\)
- as a base with HBr
- as an acid with \(\ce{OH^-}\)
- Answer a
-
\(\ce{H2PO4-}(aq)+\ce{HBr}(aq)\rightleftharpoons \ce{H3PO4}(aq)+\ce{Br-}(aq)\)
- Answer b
-
\(\ce{H2PO4-}(aq)+\ce{OH^-} (aq)\rightleftharpoons \ce{HPO4^2-}(aq)+ \ce{H_2O}_{(l)} \)
Summary
A compound that can donate a proton (a hydrogen ion) to another compound is called a Brønsted-Lowry acid. The compound that accepts the proton is called a Brønsted-Lowry base. The species remaining after a Brønsted-Lowry acid has lost a proton is the conjugate base of the acid. The species formed when a Brønsted-Lowry base gains a proton is the conjugate acid of the base. Thus, an acid-base reaction occurs when a proton is transferred from an acid to a base, with formation of the conjugate base of the reactant acid and formation of the conjugate acid of the reactant base. Amphiprotic species can act as both proton donors and proton acceptors. Water is an important amphiprotic species.
Glossary
- acid ionization
- reaction involving the transfer of a proton from an acid to water, yielding hydronium ions and the conjugate base of the acid
- amphiprotic
- species that may either gain or lose a proton in a reaction
- amphoteric
- species that can act as either an acid or a base
- base ionization
- reaction involving the transfer of a proton from water to a base, yielding hydroxide ions and the conjugate acid of the base
- Brønsted-Lowry acid
- proton donor
- Brønsted-Lowry base
- proton acceptor
- conjugate acid
- substance formed when a base gains a proton
- conjugate base
- substance formed when an acid loses a proton
Contributors and Attributions
Paul Flowers (University of North Carolina - Pembroke), Klaus Theopold (University of Delaware) and Richard Langley (Stephen F. Austin State University) with contributing authors. Textbook content produced by OpenStax College is licensed under a Creative Commons Attribution License 4.0 license. Download for free at http://cnx.org/contents/85abf193-2bd...a7ac8df6@9.110).

