9.1: Covalent Bonding Fundamentals
- Page ID
- 170028
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Learning Objectives
- Understand the relationship between potential energy and internuclear distance.
- Understand a covalent bond in terms of potential energy.
- Recognize that there are two models used to describe covalent bonding: valence bond theory and molecular orbital theory
Why are some substances chemically bonded molecules and others are an association of ions? The answer to this question depends upon the electronic structures of the atoms and nature of the chemical forces within the compounds. Although there are no sharply defined boundaries, chemical bonds are typically classified into three main types: ionic bonds, covalent bonds, and metallic bonds.
- Ionic bonds results from electrostatic forces that exist between ions of opposite charge. These bonds typically involves a metal with a nonmetal
- Covalent bonds result from the sharing of electrons between two atoms. The bonds typically involves one nonmetallic element with another
- Metallic bonds These bonds are found in solid metals (copper, iron, aluminum) with each metal bonded to several neighboring groups and bonding electrons free to move throughout the 3-dimensional structure.
In this chapter, we will focus on covalent bonding.
Covalent Bonding
A covalent bond is the sharing of electrons between two atoms. The electrons are the “glue” that keeps the two atoms together. When two atoms share electrons, the shared electrons create a region of higher electron density between the nuclei of the atoms. This concentration of negative charge pulls the nuclei together. The nuclei do not get too close to each other, however, because they repel each other. At some distance, the attraction and the repulsion balance each other, forming a stable bond.
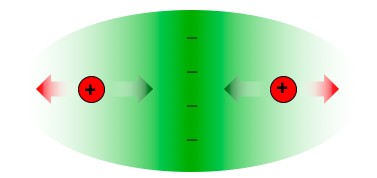
Figure \(\PageIndex{1}\): The two nuclei (red) are attracted to the high electron density in the center of the molecule (green arrows), but repelled by each other (red arrows).
Bond Energies
Fundamentally, atoms form a chemical bond because the overall energy of the bonded atoms is lower than the overall energy of the original, unbonded atoms. For example, an H2 molecule has a lower energy than two H atoms. We can show how the potential energy of two atoms depends on the distance between them by drawing a bond energy diagram. The bond energy diagram for H2 is shown below:
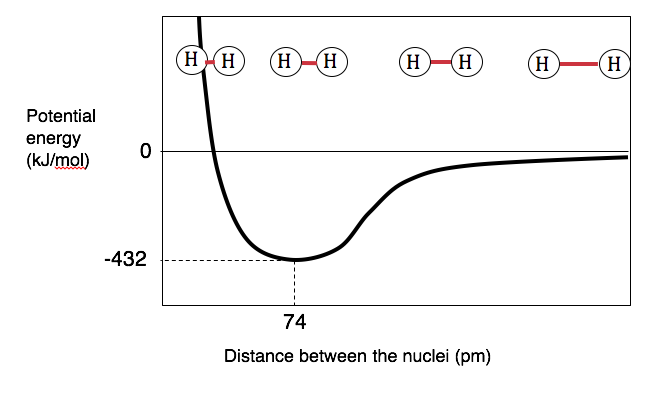
Figure \(\PageIndex{2}\): Bond energy diagram for two hydrogen atoms as a function of internuclear distance.
When charged particles interact, electrostatic forces determine the potential energy of the system. According to Coulomb's Law, the energy of the electrostatic attraction (\(E\)) between two charged particles is proportional to the magnitude of the charges and inversely proportional to the internuclear distance between the particles (\(r\)):
\[E \propto \dfrac{Q_{1}Q_{2}}{r} \label{Eq1a} \]
where each particle’s charge is represented by the symbol Q. If Q1 and Q2 have opposite signs (as an electron and a proton, where Q1 is −1 for an elecctron and Q2 is +1 for a proton), then E is negative, which means that energy is released when oppositely charged particles are brought together from an infinite distance to form an isolated pair. The opposite is true for like-charged particles. Figure \(\PageIndex{2}\) considers all of the the potential energy interactions for the two hydrogen atoms:
- proton-electron attraction (E is negative, stabilizing)
- electron-electron repulsion (E is positive, destabilizing)
- proton-proton repulsion (E is positive, destabilizing)
The total energy of the two-atom system is a sum of these these attractive and repulsive interactions. As shown by the curve in Figure \(\PageIndex{2}\), when the atoms are very far apart from one another, they have no interaction and the energy of the system is defined as zero. As the atoms are brought closer together, the stabilizing attraction between electrons and protons dominates. As a result, as atoms are brought together, energy is released and the system becomes more stable compared to the infinitely separated atoms. However, at very short internuclear distances, the repulsive electron–electron and proton-proton interactions dominate. Note that at a mid-distance, the diagram shows that the total potential energy of the system reaches a minimum, the point where the electrostatic repulsions and attractions are balanced. This distance, 74 pm, is called the bond distance (or bond length) of hydrogen. If the atoms are farther apart, the inward attraction toward the electron density pulls them closer together. If the atoms are closer together, their mutual repulsion pushes them apart. In practice, the atoms vibrate, as if they were connected by a spring.
Energy is always released when a bond is formed and correspondingly, it always requires energy to break a bond.
The graph in Figure \(\PageIndex{2}\) also shows us that the H2 molecule is 432 kJ/mol more stable than the unbonded atoms (which would have an energy of 0 kJ/mol). If we wanted to break a mole of H2 into hydrogen atoms, we would need to put in 432 kJ of energy:
H2(g) \(\rightarrow\) 2 H(g) \(\Delta\)H = 432 kJ
This energy is called the bond dissociation energy (or bond energy) of H2.
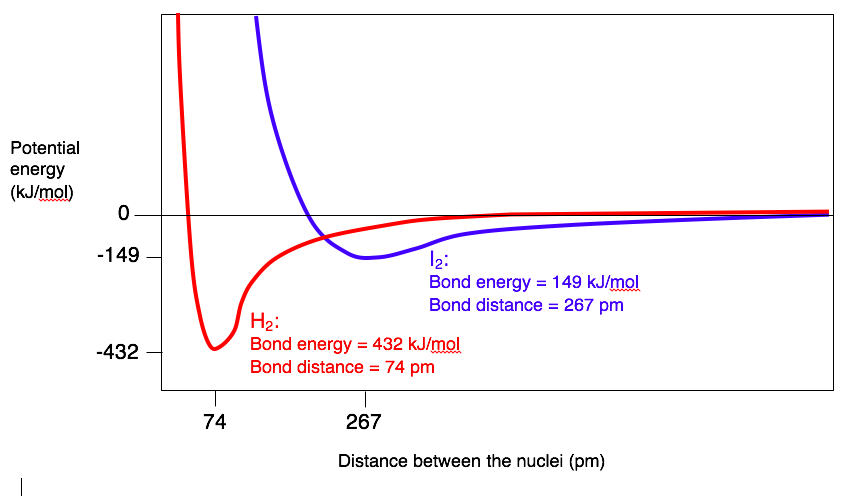
Bond energies and bond distances depend on the atoms that are bonded together. A dramatic illustration of this is shown by the graph below, which compares the bond energy diagrams of H2 and I2.
Two Models for Covalent Bonding
There are two models that we can use to describe covalent bonding and how it produces the molecules we observe in nature
Localized Electron Model: assumes that each pair of electrons in a molecule has a specific location and function. Nonbonding electron pairs are in atomic orbitals; bonding electron pairs are found where atomic orbitals from two atoms overlap.
Delocalized Electron: assumes that each pair of electrons in a molecule occupies an orbital that encompass the entire molecule. Every molecule has a set of these molecular orbitals, which are filled in the same way that atomic orbitals are filled in a single atom.
Localized Electron Model of Covalent Bonding
The valence-bond model is essentially the Lewis structure model that you have probably encountered in your introductory chemistry class, but expanded to include some of the ideas of quantum mechanics. This model is the easier of the two to apply, so we will focus most of our attention on it.The valence-bond model, sometimes referred to as the "localized electron model" of covalent bonding, is based on the following general postulates.
1) A covalent bond is the sharing of a pair of electrons between two atoms.
We typically represent this by a Lewis structure, which shows the valence electrons as dots. You should be somewhat familiar with Lewis structures from prior coursework. Detailed instructions for drawing Lewis structures are covered in a separate section.
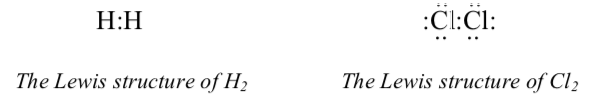
2) The bond forms when two atomic orbitals overlap.
For example, the bond in H2 is formed when the 1s orbitals of the two atoms overlap.

3) Each pair of electrons that is not involved in bonding is localized on one atom.
In the Lewis structure of Cl2 above, six of the seven pairs of electrons are non-bonding. Each pair is localized on one atom: three pairs on the left-hand Cl and three on the right-hand Cl.
4) The shape of a molecule is determined by the repulsion of electrons in the valence shells of the atoms.
For example, in BeF2, the atoms line up because the two bonding electron pairs repel each other and stay on opposite sides of the Be atom.

Delocalized Electron Model of Covalent Bonding
The delocalized electron model model is much closer to reality than the localized electron model model. It accounts for delocalized electrons, which Lewis structures don’t deal with well, and it allows us to explain some observations that Lewis structures cannot account for, such as energetic and magnetic behavior of molecules. The delocalized electron model is encompassed in Molecular Orbital Theory. This theory is based on the following assumptions:
- Every molecule has a set of orbitals.
- Each of these molecular orbitals encompasses the entire molecule.
- The rules for filling these orbitals are the same as they are for filling atomic orbitals: only 2 electrons per orbital, the lowest energy orbitals fill first, and electrons spread themselves out when they are in equal-energy orbitals.
- We can find the approximate shapes of molecular orbitals by adding wave functions for atomic orbitals.
Although more accurate, this model requires a more sophisticated mathematical treatment. We will introduce the core ideas by considering diatomic molecules.

