3.6: Reaction Stoichiometry
- Page ID
- 169957
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Learning Objectives
- To balance equations that describe reactions in solution.
- To calculate the quantities of compounds produced or consumed in a chemical reaction.
- To solve quantitative problems involving the stoichiometry of reactions in solution.
A balanced chemical equation gives the identity of the reactants and the products as well as the accurate number of molecules or moles of each that are consumed or produced. Stoichiometry is a collective term for the quantitative relationships between the masses, the numbers of moles, and the numbers of particles (atoms, molecules, and ions) of the reactants and the products in a balanced chemical equation. A stoichiometric quantity is the amount of product or reactant specified by the coefficients in a balanced chemical equation. This section describes how to use the stoichiometry of a reaction to answer questions like the following: How much oxygen is needed to ensure complete combustion of a given amount of isooctane? (This information is crucial to the design of nonpolluting and efficient automobile engines.) How many grams of pure gold can be obtained from a ton of low-grade gold ore? (The answer determines whether the ore deposit is worth mining.) If an industrial plant must produce a certain number of tons of sulfuric acid per week, how much elemental sulfur must arrive by rail each week?
All these questions can be answered using the concepts of the mole, molar and formula masses, and solution concentrations, along with the coefficients in the appropriate balanced chemical equation.
Stoichiometry Problems
When carrying out a reaction in either an industrial setting or a laboratory, it is easier to work with masses of substances than with the numbers of molecules or moles. The general method for converting from the mass of any reactant or product to the mass of any other reactant or product using a balanced chemical equation is outlined in and described in the following text.
Steps in Converting between Masses of Reactant and Product
- Convert the mass of one substance (substance A) to the corresponding number of moles using its molar mass.
- From the balanced chemical equation, obtain the number of moles of another substance (B) from the number of moles of substance A using the appropriate mole ratio (the ratio of their coefficients).
- Convert the number of moles of substance B to mass using its molar mass. It is important to remember that some species are present in excess by virtue of the reaction conditions. For example, if a substance reacts with the oxygen in air, then oxygen is in obvious (but unstated) excess.
Converting amounts of substances to moles—and vice versa—is the key to all stoichiometry problems, whether the amounts are given in units of mass (grams or kilograms), weight (pounds or tons), or volume (liters or gallons).
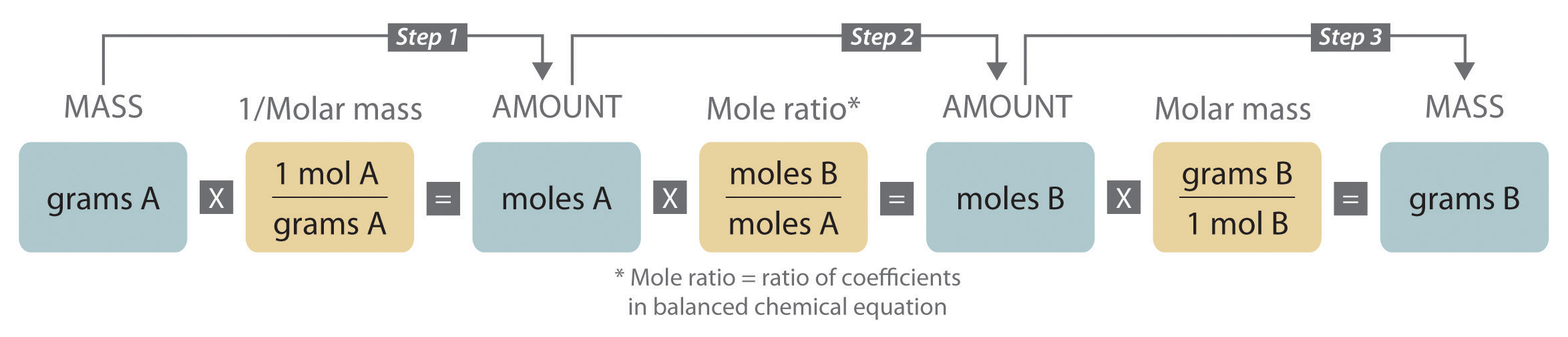
To illustrate this procedure, consider the combustion of glucose. Glucose reacts with oxygen to produce carbon dioxide and water:
\[ C_6H_{12}O_6 (s) + 6 O_2 (g) \rightarrow 6 CO_2 (g) + 6 H_2O (l) \tag{3.6.1}\]
Just before a chemistry exam, suppose a friend reminds you that glucose is the major fuel used by the human brain. You therefore decide to eat a candy bar to make sure that your brain does not run out of energy during the exam (even though there is no direct evidence that consumption of candy bars improves performance on chemistry exams). If a typical 2 oz candy bar contains the equivalent of 45.3 g of glucose and the glucose is completely converted to carbon dioxide during the exam, how many grams of carbon dioxide will you produce and exhale into the exam room?
The initial step in solving a problem of this type is to write the balanced chemical equation for the reaction. Inspection shows that it is balanced as written, so the strategy outlined above can be adapted as follows:
1. Use the molar mass of glucose (to one decimal place, 180.2 g/mol) to determine the number of moles of glucose in the candy bar:
\[ moles \, glucose = 45.3 \, g \, glucose \times {1 \, mol \, glucose \over 180.2 \, g \, glucose } = 0.251 \, mol \, glucose \]
2. According to the balanced chemical equation, 6 mol of CO2 is produced per mole of glucose; the mole ratio of CO2 to glucose is therefore 6:1. The number of moles of CO2 produced is thus
\[ moles \, CO_2 = mol \, glucose \times {6 \, mol \, CO_2 \over 1 \, mol \, glucose } \]
\[ = 0.251 \, mol \, glucose \times {6 \, mol \, CO_2 \over 1 \, mol \, glucose } \]
\[ = 1.51 \, mol \, CO_2 \]
3. Use the molar mass of CO2 (44.010 g/mol) to calculate the mass of CO2 corresponding to 1.51 mol of CO2:These operations can be summarized as follows:
\[ 45.3 \, g \, glucose \times {1 \, mol \, glucose \over 180.2 \, g \, glucose} \times {6 \, mol \, CO_2 \over 1 \, mol \, glucose} \times {44.010 \, g \, CO_2 \over 1 \, mol \, CO_2} = 66.4 \, g \, CO_2 \]
Discrepancies between the two values are attributed to rounding errors resulting from using stepwise calculations in steps 1–3. (Remember that you should generally carry extra significant digits through a multistep calculation to the end to avoid this!) This amount of gaseous carbon dioxide occupies an enormous volume—more than 33 L. Similar methods can be used to calculate the amount of oxygen consumed or the amount of water produced.
The balanced chemical equation was used to calculate the mass of product that is formed from a certain amount of reactant. It can also be used to determine the masses of reactants that are necessary to form a certain amount of product or, as shown in Example \(\PageIndex{1}\), the mass of one reactant that is required to consume a given mass of another reactant.
Example \(\PageIndex{1}\): The US Space Shuttle
The combustion of hydrogen with oxygen to produce gaseous water is extremely vigorous, producing one of the hottest flames known. Because so much energy is released for a given mass of hydrogen or oxygen, this reaction was used to fuel the NASA (National Aeronautics and Space Administration) space shuttles, which have recently been retired from service. NASA engineers calculated the exact amount of each reactant needed for the flight to make sure that the shuttles did not carry excess fuel into orbit. Calculate how many tons of hydrogen a space shuttle needed to carry for each 1.00 tn of oxygen (1 tn = 2000 lb).

The US space shuttle Discovery during liftoff. The large cylinder in the middle contains the oxygen and hydrogen that fueled the shuttle’s main engine.
Given: reactants, products, and mass of one reactant
Asked for: mass of other reactant
Strategy:
- Write the balanced chemical equation for the reaction.
- Convert mass of oxygen to moles. From the mole ratio in the balanced chemical equation, determine the number of moles of hydrogen required. Then convert the moles of hydrogen to the equivalent mass in tons.
Solution:
We use the same general strategy for solving stoichiometric calculations as in the preceding example. Because the amount of oxygen is given in tons rather than grams, however, we also need to convert tons to units of mass in grams. Another conversion is needed at the end to report the final answer in tons.
A We first use the information given to write a balanced chemical equation. Because we know the identity of both the reactants and the product, we can write the reaction as follows:
\[ H_2 (g) + O_2 (g) \rightarrow H_2O (g) \]
This equation is not balanced because there are two oxygen atoms on the left side and only one on the right. Assigning a coefficient of 2 to both H2O and H2 gives the balanced chemical equation:
\[ 2 H_2 (g) + O_2 (g) \rightarrow 2 H_2O (g) \]
Thus 2 mol of H2 react with 1 mol of O2 to produce 2 mol of H2O.
1. B To convert tons of oxygen to units of mass in grams, we multiply by the appropriate conversion factors:
Using the molar mass of O2 (32.00 g/mol, to four significant figures), we can calculate the number of moles of O2 contained in this mass of O2:
\[ mol \, O_2 = 9.07 \times 10^5 \, g \, O_2 \times {1 \, mol \, O_2 \over 32.00 \, g \, O_2} = 2.83 \times 10^4 \, mol \, O_2 \]
2. Now use the coefficients in the balanced chemical equation to obtain the number of moles of H2 needed to react with this number of moles of O2:
\[ mol \, H_2 = mol \, O_2 \times {2 \, mol \, H_2 \over 1 \, mol \, O_2} \]
\[ = 2.83 \times 10^4 \, mol \, O_2 \times {2 \, mol \, H_2 \over 1 \, mol \, O_2} = 5.66 \times 10^4 \, mol \, H_2 \]
3. The molar mass of H2 (2.016 g/mol) allows us to calculate the corresponding mass of H2:
\[mass \, of \, H_2 = 5.66 \times 10^4 \, mol \, H_2 \times {2.016 \, g \, H_2 \over mol \, H_2} = 1.14 \times 10^5 \, g \, H_2 \]
Finally, convert the mass of H2 to the desired units (tons) by using the appropriate conversion factors:
\[ tons \, H_2 = 1.14 \times 10^5 \, g \, H_2 \times {1 \, lb \over 453.6 \, g} \times {1 \, tn \over 2000 \, lb} = 0.126 \, tn \, H_2 \]
The space shuttle had to be designed to carry 0.126 tn of H2 for each 1.00 tn of O2. Even though 2 mol of H2 are needed to react with each mole of O2, the molar mass of H2 is so much smaller than that of O2 that only a relatively small mass of H2 is needed compared to the mass of O2.
Exercise \(\PageIndex{1}\): Roasting Cinnabar
Cinnabar, (or Cinnabarite) \(HgS\) is the common ore of mercury. Because of its mercury content, cinnabar can be toxic to human beings; however, because of its red color, it has also been used since ancient times as a pigment.

Alchemists produced elemental mercury by roasting cinnabar ore in air:
\[ HgS (s) + O_2 (g) \rightarrow Hg (l) + SO_2 (g) \]
The volatility and toxicity of mercury make this a hazardous procedure, which likely shortened the life span of many alchemists. Given 100 g of cinnabar, how much elemental mercury can be produced from this reaction?
- Answer
-
86.2 g
Exercise \(\PageIndex{2}\): Copper Reacts with Nitric Acid
Copper reacts with nitric acid as follows:
\[ \ce{ Cu + 4 HNO3 -> Cu(NO3)2 + 2NO2 + 2H2O}\]
A chemist mixes 4.523 g of copper and 11.716 g of nitric acid.
a) Which reactant (if any) will remain if the reaction goes to completion, and how many grams of that reactant will be left over?
b) What mass of nitrogen dioxide will be formed, assuming 100% yield?
c) If the chemist isolates 8.333 g of copper(II) nitrate, what is the percent yield in this experiment?
- Answer
-
a) First, you must determine the limiting reactant, which can be done in several ways. One simple option is to calculate the mass of one of the products based on each reactant; the reactant that gives the smaller mass of product is the limiting reactant. This method is convenient here because if we choose NO2 as our product, we will also get the answer to part b of the problem.
If we use all of the Cu, we will form…
\[4.523 \mathrm{g} \mathrm{Cu} \times \frac{1 \mathrm{mol} \mathrm{Cu}}{63.55 \mathrm{g} \mathrm{Cu}} \times \frac{2 \mathrm{mol} \mathrm{NO}_{2}}{1 \mathrm{mol} \mathrm{Cu}} \times \frac{46.01 \mathrm{g} \mathrm{NO}_{2}}{1 \mathrm{mol} \mathrm{NO}_{2}}=6.549 \mathrm{g} \mathrm{NO}_{2} \]
If we use all of the HNO3, we will form…
\[11.716 \mathrm{g} \mathrm{HNO}_{3} \times \frac{1 \mathrm{mol} \mathrm{HNO}_{3}}{63.018 \mathrm{g} \mathrm{HNO}_{3}} \times \frac{2 \mathrm{mol} \mathrm{NO}_{2}}{4 \mathrm{mol} \mathrm{HNO}_{3}} \times \frac{46.01 \mathrm{g} \mathrm{NO}_{2}}{1 \mathrm{mol} \mathrm{NO}_{2}}=4.277 \mathrm{g} \mathrm{NO}_{2} \]
Since the HNO3 gives less product, HNO3 is the limiting reactant and will be completely consumed in the reaction. Therefore, Cu will remain after the reaction goes to completion. To calculate the remaining mass of Cu, we must first determine how much Cu is consumed, given that all of the HNO3 reacts:
\[11.716 \mathrm{g} \mathrm{HNO}_{3} \times \frac{1 \mathrm{mol} \mathrm{HNO}_{3}}{63.018 \mathrm{g} \mathrm{HNO}_{3}} \times \frac{1 \mathrm{mol} \mathrm{Cu}}{4 \mathrm{mol} \mathrm{HNO}_{3}} \times \frac{63.55 \mathrm{g} \mathrm{Cu}}{1 \mathrm{mol} \mathrm{Cu}}=2.954 \mathrm{gCu}\]
The remaining mass of Cu is therefore 4.523 g – 2.954 g = 1.569 g.
b) As we found in part a, we will form 4.277 g of NO2.
c) Calculate the mass of Cu(NO3)2 that should be formed (the theoretical yield):
\[ \begin{aligned} 11.716 \mathrm{g} \mathrm{HNO}_{3} \times \frac{1 \mathrm{mol} \mathrm{HNO}_{3}}{63.018 \mathrm{g} \mathrm{HNO}_{3}} \times \frac{1 \mathrm{mol} \mathrm{Cu}\left(\mathrm{NO}_{3}\right)_{2}}{4 \mathrm{mol} \mathrm{HNO}_{3}} \times & \frac{187.57 \mathrm{g} \mathrm{Cu}\left(\mathrm{NO}_{3}\right)_{2}}{1 \mathrm{mol} \mathrm{Cu}\left(\mathrm{NO}_{3}\right)_{2}} \\ &=8.718 \mathrm{g} \mathrm{Cu}\left(\mathrm{NO}_{3}\right)_{2} \end{aligned}\]
The percent yield is: \(\frac{8.333 g}{8.718g} \times 100 \%=95.58 \%\)
Exercise \(\PageIndex{3}\): Working with Ice Tables
Stoichiometry problems can be approached by using "ICE" tables to organize your thoughts. ICE stands for "Initial moles", "Change in moles", and "End number of moles." Complete the following ICE tables. For each of these tables, the relevant reaction is:
\[ \ce{ 2C2H6 + 7O2 -> 4CO2 + 6H2O} \]
|
a) |
2 C2H6 |
7 O2 |
4 CO2 |
6 H2O |
|
Initial |
2.474 mol |
5.131 mol |
0 mol |
0 mol |
|
Change |
||||
|
End |
- Answer (a)
-
As in any stoichiometry problem where you are given the numbers of moles of each reactant, you must first determine the limiting reactant. In an ICE table, a simple way to do this (if it isn’t immediately obvious) is to assume that the first reactant is completely used up and calculate the number of moles of the other reactant(s) that will be consumed. Then check to be sure that you aren’t using more moles than you started with.
For example, let’s assume that all of the C2H6 is consumed. How much O2 will we use?
\( 2.474 \mathrm{mol} \mathrm{C}_{2} \mathrm{H}_{6} \times \frac{7 \mathrm{mol} \mathrm{O}_{2}}{2 \mathrm{mol} \mathrm{C}_{2} \mathrm{H}_{6}}=8.659 \mathrm{mol} \mathrm{O}_{2}\) will be used
Inserting these numbers into the table gives us:
2 C2H6
7 O2
4 CO2
6 H2O
Initial
2.474 mol
5.131 mol
0 mol
0 mol
Change
– 2.474 mol
– 8.659 mol
End
0 mol
-3.528 mol
We end up with a negative amount of oxygen, because we used more moles than we were given. This is obviously impossible, so O2 must be the limiting reactant. Once we know this, it is straightforward to complete the table. The amount of C2H6 that we use up is:
\(5.131 \mathrm{mol} \mathrm{O}_{2} \times \frac{2 \mathrm{mol} \mathrm{C}_{2} \mathrm{H}_{6}}{7 \mathrm{mol} \mathrm{O}_{2}}=1.466 \mathrm{mol} \mathrm{C}_{2} \mathrm{H}_{6}\)
The amount of CO2 that we make is:
\(5.131 \mathrm{mol} \mathrm{O}_{2} \times \frac{4 \mathrm{mol} \mathrm{CO}_{2}}{7 \mathrm{mol} \mathrm{O}_{2}}=2.932 \mathrm{mol} \mathrm{CO}_{2}\)
The amount of H2O that we make is calculated similarly. The completed table looks like this:
2 C2H6
7 O2
4 CO2
6 H2O
Initial
2.474 mol
5.131 mol
0 mol
0 mol
Change
– 1.466 mol
– 5.131 mol
+ 2.932 mol
+ 4.398 mol
End
1.008 mol
0 mol
2.932 mol
4.398 mol
|
b) |
2 C2H6 |
7 O2 |
4 CO2 |
6 H2O |
|
Initial |
0.0518 mol |
0.2331 mol |
0.0489 mol |
0 mol |
|
Change |
||||
|
End |
- Answer (b)
-
In this table, we have some CO2 (a product) in the initial mixture. This does not affect any of our calculations; the CO2 we make in the reaction just gets added to this initial amount.
In this case, the limiting reactant is C2H6. You can determine this using the same method we used in part a. The completed table looks like this:
2 C2H6
7 O2
4 CO2
6 H2O
Initial
0.0518 mol
0.2331 mol
0.0489 mol
0 mol
Change
– 0.0518 mol
–0.1813 mol
+ 0.1036 mol
+ 0.1554 mol
End
0 mol
0.0518 mol
0.1525 mol
0.1554 mol
Exercise \(\PageIndex{4}\): Stoichiometry and Algebraic Variables
The mole-to-mol ratios in a balanced chemical equation are at the heart of reaction stoichiometry. In fact, you can consider the amounts of reactants mixed together in terms of variable and still work through a stoichiometry problem. We will again use "ICE" tables to organize our thoughts. Complete the following ICE tables. For each of these tables, the relevant reaction is:
\[ \ce{2C2H6 + 7O2 -> 4CO2 + 6H2O}\]
|
a) |
2 C2H6 |
7 O2 |
4 CO2 |
6 H2O |
|
Initial |
x mol |
2x mol |
0 mol |
0 mol |
|
Change |
||||
|
End |
- Answer (a)
-
What do we do with variables?? We treat them the same we treat numbers! Let’s assume that all of the C2H6 is consumed, just as did in part a. How much O2 will we use?
\[x \operatorname{mol} \mathrm{C}_{2} \mathrm{H}_{6} \times \frac{7 \mathrm{mol} \mathrm{O}_{2}}{2 \mathrm{mol} \mathrm{C}_{2} \mathrm{H}_{6}}=3.5 x \mathrm{mol} \mathrm{O}_{2} \text { will be used } \]
If we use x mol of C2H6 and 3.5x mol of O2, our table will look like this:
2 C2H6
7 O2
4 CO2
6 H2O
Initial
x mol
2x mol
0 mol
0 mol
Change
– x mol
– 3.5x mol
End
0 mol
-1.5x mol
As in part a of Exercise \(\PageIndex{3}\), if we use all of the C2H6, we use more O2 than we actually have. Therefore, O2 must be the limiting reactant. The amount of C2H6 we use is:
\[2 x \operatorname{mol} \mathrm{O}_{2} \times \frac{2 \operatorname{mol} \mathrm{C}_{2} \mathrm{H}_{6}}{7 \mathrm{mol} \mathrm{O}_{2}}=\frac{4}{7} x \mathrm{mol} \mathrm{C}_{2} \mathrm{H}_{6}=0.5714 x \mathrm{mol} \mathrm{C}_{2} \mathrm{H}_{6}\]
The amount of CO2 we make is:
\[2 x \operatorname{mol} \mathrm{O}_{2} \times \frac{4 \mathrm{mol} \mathrm{CO}_{2}}{7 \mathrm{mol} \mathrm{O}_{2}}=\frac{8}{7} x \mathrm{mol} \mathrm{CO}_{2}=1.1429 x \mathrm{mol} \mathrm{CO}_{2}\]
The amount of H2O we make is:
\[2 x \operatorname{mol} \mathrm{O}_{2} \times \frac{6 \mathrm{mol} \mathrm{H}_{2} \mathrm{O}}{7 \mathrm{mol} \mathrm{O}_{2}}=\frac{12}{7} x \mathrm{mol} \mathrm{H}_{2} \mathrm{O}=1.7143 x \mathrm{mol} \mathrm{H}_{2} \mathrm{O}\]
The completed table looks like this:
2 C2H6
7 O2
4 CO2
6 H2O
Initial
x mol
2x mol
0 mol
0 mol
Change
– 0.5714x mol
– 2x mol
+ 1.1429x mol
+ 1.7143x mol
End
0.4286x mol
0 mol
1.1429x mol
1.7143x mol
(You can also express all of the moles as fractions. The final numbers will be 3/7x mol C2H6, 8/7x mol CO2, and 12/7x mol H2O.)
For part b, complete the table assuming that C2H6 is the limiting reactant.
|
b) |
2 C2H6 |
7 O2 |
4 CO2 |
6 H2O |
|
Initial |
x mol |
y mol |
0 mol |
0 mol |
|
Change |
||||
|
End |
- Answer (b)
-
We are told that C2H6 is the limiting reactant, so all of the amounts in the “change” row will be based on the initial moles of C2H6. The amount of O2 that we use is:
\[x \operatorname{mol} \mathrm{C}_{2} \mathrm{H}_{6} \times \frac{7 \mathrm{mol} \mathrm{O}_{2}}{2 \mathrm{mol} \mathrm{C}_{2} \mathrm{H}_{6}}=3.5 x \mathrm{mol} \mathrm{O}_{2}\]
The amount of CO2 we make will be:
\[ x \operatorname{mol} \mathrm{C}_{2} \mathrm{H}_{6} \times \frac{4 \mathrm{mol} \mathrm{CO}_{2}}{2 \mathrm{mol} \mathrm{C}_{2} \mathrm{H}_{6}}=2 x \mathrm{mol} \mathrm{CO}_{2}\]
The amount of H2O we make will be:
\[x \operatorname{mol} \mathrm{C}_{2} \mathrm{H}_{6} \times \frac{6 \mathrm{mol} \mathrm{H}_{2} \mathrm{O}}{2 \mathrm{mol} \mathrm{C}_{2} \mathrm{H}_{6}}=3 x \mathrm{mol} \mathrm{H}_{2} \mathrm{O}\]
The completed table looks like this:
2 C2H6
7 O2
4 CO2
6 H2O
Initial
x mol
y mol
0 mol
0 mol
Change
– x mol
– 3.5x mol
+ 2x mol
+ 3x mol
End
0 mol
y – 3.5x mol
2x mol
3x mol
For part c, complete the table assuming that O2 is the limiting reactant.
|
c) |
2 C2H6 |
7 O2 |
4 CO2 |
6 H2O |
|
Initial |
x mol |
y mol |
0 mol |
0 mol |
|
Change |
||||
|
End |
- Answer (c)
-
Now we are told that O2 is the limiting reactant. You should be able to figure out the amounts in the “change” row using the same method we used in parts b and c. The completed table looks like this:
2 C2H6
7 O2
4 CO2
6 H2O
Initial
x mol
y mol
0 mol
0 mol
Change
– 0.2857y mol
– y mol
+ 0.5714y mol
+ 0.8571y mol
End
x – 0.2857y mol
0 mol
0.5714y mol
0.8571y mol
As in part b, you can also express the amounts as fractions if you choose. The final numbers will be x – 2/7y mol C2H6, 4/7y mol CO2, and 6/7y mol H2O.
Summary
The coefficients in the balanced chemical equation tell how many moles of reactants are needed and how many moles of product can be produced.

