10.3: Urea Cycle
- Page ID
- 234049
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)The fate of the amino group: the urea cycle
Deamination of amino acids results in the production of ammonium (NH4+). Ammonium is an extremely toxic base and its accumulation in the body would quickly be fatal. However, the liver contains a system of carrier molecules and enzymes which quickly converts the ammonia (and carbon dioxide) into urea. This is called the urea cycle.
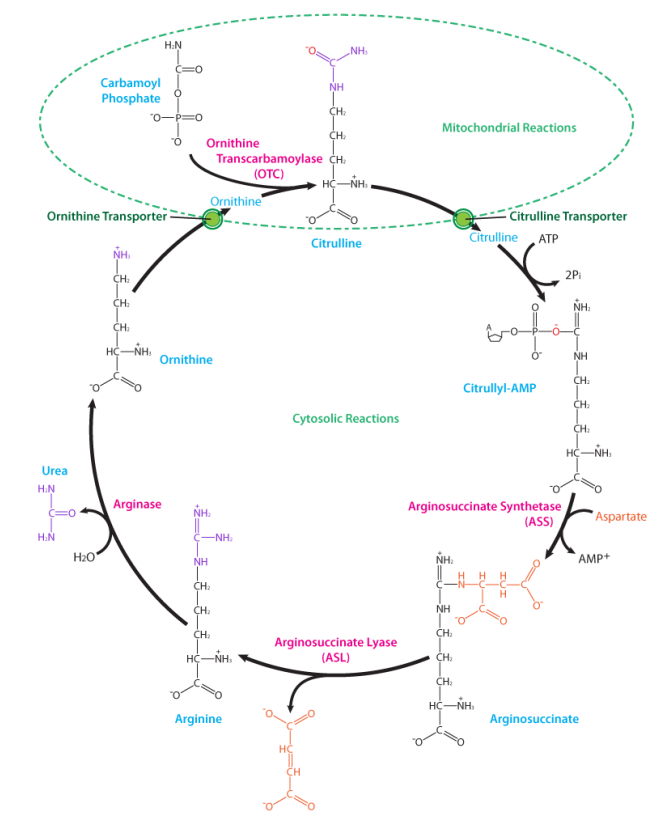
The cycle scavenges free ammonia (as ammonium ion) which is toxic if it accumulates. The capture reaction also requires ATP, and bicarbonate, and the product is carbamoyl phosphate. The reaction is catalyzed by the enzyme carbamoyl phosphate synthetase:
This molecule is combined with the non-protein amino acid known as ornithine to make another non-protein amino acid known as citrulline. Addition of aspartate to citrulline creates argninosuccinate, which splits off a fumarate, creating arginine (a source of arginine for the body). If arginine is not needed, it can be hydrolyzed to yield urea (excreted) and ornithine, thus completing the cycle. The first two reactions described here occur in the mitochondrion and the remaining ones occur in the cytoplasm. Molecules of the urea cycle intersecting other pathways include fumarate (citric acid cycle), aspartate (amino acid metabolism), arginine (amino acid metabolism), and ammonia (amino acid metabolism).
After urea is formed, it is excreted in the urine. An adult excretes 20–30 g of urea in the urine daily.
The overall equation of the urea cycle is:
Uric acid
Humans also excrete a second nitrogenous waste, uric acid. It is the product of nucleic acid, not protein, metabolism. It is produced within peroxisomes, and it is excreted in the urine, or reabsorbed by kidneys to regulate blood levels. Uric acid is a potent antioxidant and thus can protect cells from damage by reactive oxygen species (ROS). In human blood plasma, the reference range of uric acid is typically 3.4–7.2 mg per 100 mL for men, and 2.4–6.1 mg per 100 mL for women. Uric acid concentrations in blood plasma above and below the normal range are known as, respectively, hyperuricemia and hypouricemia.
Uric acid is only slightly soluble in water and easily precipitates out of solution forming needle-like crystals of sodium urate. These contribute to the formation of kidney stones and produce the excruciating pain of gout when deposited in the joints.
Uric acid is only slightly soluble in water and easily precipitates out of solution forming needle-like crystals of sodium urate. These contribute to the formation of kidney stones and produce the excruciating pain of gout when deposited in the joints. The concentration of uric acid is 100-times greater in the cytosol than in the extracellular fluid. So when lethally-damaged cells release their contents, crystals of uric acid form in the vicinity.
So the risk of kidney stones and gout may be the price we pay for the antioxidant protection provided by uric acid.
Most mammals have an enzyme - uricas - for breaking uric acid down into a soluble product. However, during the evolution of great apes and humans, the gene encoding uricase became inactive. A predisposition to gout is our legacy.
Contributors
- Kevin Ahern & Indira Rajagopal. Biochemistry Free & Easy. LibreTexts content adapted under CC BY-NC-SA 3.0 license.
- John W. Kimball. Biology. LibreTexts content adopted under Creative Commons Attribution 3.0 Unported (CC BY 3.0) license and made possible by funding from The Saylor Foundation.
- Template:ContribAhern
- Wikipedia contributors. (2021, April 8). Uric acid. In Wikipedia, The Free Encyclopedia. Retrieved 20:30, April 10, 2021, from https://en.wikipedia.org/w/index.php?title=Uric_acid&oldid=1016645551


