5.7: Regulation of Enzymatic activity
- Page ID
- 279157
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Enzymes can be regulated by changing the activity of a preexisting enzyme or changing the amount of an enzyme.
A. Changing the activity of a pre-existing enzyme
The quickest way to modulate the activity of an enzyme is to alter the activity of an enzyme that already exists in the cell. The list below, illustrated in the following figure, gives common ways to regulate enzyme activity
- Substrate availability: Substrates (reactants) bind to enzymes with a characteristic affinity, characterized by a kinetic parameter Km. If the actual concentration of a substrate in a cell is much less than the Km, the activity of the enzyme is very low. If the substrate concentration is much greater than Km, the enzyme active site is saturated with substrate and the enzyme is maximally active.
- Product inhibition: A product of an enzyme-catalyzed reaction often resembles a starting reactant, so it should be clear that the product should also bind to the active site, albeit probably with lower affinity (competitive inhibitor). Under conditions in which the product of a reaction is present in high concentration, it would be energetically advantageous to the cell if no more product was synthesized. Product inhibition is hence commonly observed. Likewise it be energetically advantageous to a cell if the end product of an entire pathway could likewise bind to the initial enzyme in the pathways and inhibit it, allowing the whole pathway to be inhibited. This type of feedback inhibition is commonly observed.
- Allosteric regulation: Molecules that bind to sites on target enzymes other than the active site (allosteric sites) can regulate the activity of the target enzyme (in the same way as noncompetitive inhibitor). These molecules can be structurally dissimilar to those that bind at the active site. They do so my conformational changes which can either activate or inhibit the target enzyme's activity. One type of very common allosteric regulation is feedback inhibition. In this type of allosteric regulation, the end product of a metabolic pathways inhibits the first enzyme in the pathway by binding to an allosteric site.
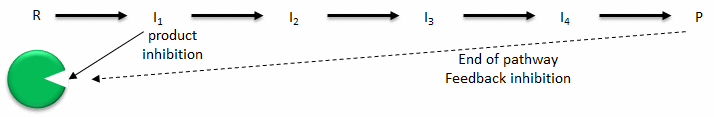
Figure: Feedback inhibition vs product inhibition.
- Covalent modification: Many if not most proteins are subjected to post-translational modifications which can affect enzyme activity through local or global shape changes, by promoting or inhibiting binding interaction of substrates and allosteric regulators, and even by changing the location of the protein within the cell. Proteins may be phosphorylated, acetylated, methylated, sulfated, glycosylated, hydroxylated,etc., often in a reversible fashion. Some of these modifications are reversible. Regulation by phosphorylation through the action of kinases, and dephosphorylation by phosphates is extremely common. Control of phosphorylation state is mediated through signal transduction process starting at the cell membrane, leading to the activation or inhibition of protein kinases and phosphatases within the cell.
B. Changing the amount of an enzyme (genetic control)
Another and less immediate but longer duration method to modulate the activity of an enzyme is to alter the activity of an enzyme that already exists in the cell. The list below, illustrated in the following figure, shows way in which enzyme concentration is regulated.
- Alternation in the transcription of enzyme's gene: Extracellular signal (hormones, neurotransmitters, etc) can lead to signal transductions responses and ultimate activation or inhibition of the transcription of the gene for a protein enzyme. These changes result from recruitment of transcription factors (proteins) to DNA sequences that regulate transcription of the enzyme gene.
- Degradation of messenger RNA for the enzyme: The levels of messenger RNA for a protein will directly determine the amount of that protein synthesized. Small inhibitor RNAs, derived from microRNA molecules transcribed from cellular DNA, can bind to specific sequences in the mRNA of a target enzyme. The resulting double-stranded RNA complex recruits an enzyme (Dicer) that cleaves the complex with the effect of decreasing translation of the protein enzyme from its mRNA.
- Post-translational changes: Once a protein enzymes is translated from its mRNA, it can undergo a changes to affect enzyme levels. Some proteins are synthesized in a "pre-form which must be cleaved in a targeted and limited fashion by proteases to active the protein enzyme (proteolytic activation). This type of enzymes are called zymogens. The precursor is often called a proenyzme. Examples of zymogens are enzymes involved in protein degradation (proteases) and programmed cell death (apoptosis). Some proteins are not fully folded and must bind to other factors in the cell to adopt a catalytically active form. Finally, fully active protein can be fully proteolyzed by the proteasome, a complex within cells, or in lysosomes, which are organelles within cells containing proteolytic enzymes.

Contributors and Attributions
Wikipedia contributors. (2021, May 6). Zymogen. In Wikipedia, The Free Encyclopedia. Retrieved 18:51, May 17, 2021, from https://en.wikipedia.org/w/index.php?title=Zymogen&oldid=1021692037


